Case-only designs in pharmacoepidemiology: a systematic review
- PMID: 23166668
- PMCID: PMC3500300
- DOI: 10.1371/journal.pone.0049444
Case-only designs in pharmacoepidemiology: a systematic review
Abstract
Background: Case-only designs have been used since late 1980's. In these, as opposed to case-control or cohort studies for instance, only cases are required and are self-controlled, eliminating selection biases and confounding related to control subjects, and time-invariant characteristics. The objectives of this systematic review were to analyze how the two main case-only designs - case-crossover (CC) and self-controlled case series (SCCS) - have been applied and reported in pharmacoepidemiology literature, in terms of applicability assumptions and specificities of these designs.
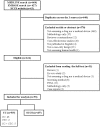
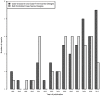
Methodology/principal findings: We systematically selected all reports in this field involving case-only designs from MEDLINE and EMBASE up to September 15, 2010. Data were extracted using a standardized form. The analysis included 93 reports 50 (54%) of CC and 45 (48%) SCCS, 2 reports combined both designs. In 12 (24%) CC and 18 (40%) SCCS articles, all applicable validity assumptions of the designs were fulfilled, respectively. Fifty (54%) articles (15 CC (30%) and 35 (78%) SCCS) adequately addressed the specificities of the case-only analyses in the way they reported results.
Conclusions/significance: Our systematic review underlines that implementation of CC and SCCS designs needs to be more rigorous with regard to validity assumptions, as well as improvement in results reporting.
Conflict of interest statement
Figures
References
-
- Maclure M (1991) The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 133: 144–53. - PubMed
-
- Suissa S (1995) The case-time-control design. Epidemiology 6: 248–53. - PubMed
-
- Farrington CP (1995) Relative incidence estimation from case series for vaccine safety evaluation. Biometrics 51: 228–35. - PubMed
-
- Farrington CP (1993) Estimation of vaccine effectiveness using the screening method. Int J Epidemiol 22: 742–6. - PubMed
-
- Petri H, de Vet HC, Naus J, Urquhart J (1988) Prescription sequence analysis: a new and fast method for assessing certain adverse reactions of prescription drugs in large populations. Stat Med 7: 1171–5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources