The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum
- PMID: 23166686
- PMCID: PMC3498113
- DOI: 10.1371/journal.pone.0049495
The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum
Abstract
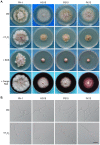
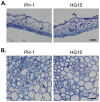
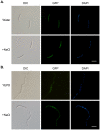
Fusarium head blight (FHB) caused by Fusarium graminearum is a destructive disease of wheat and barley worldwide. In a previous study of systematic characterization of protein kinase genes in F. graminearum, mutants of three putative components of the osmoregulation MAP kinase pathway were found to have distinct colony morphology and hyphal growth defects on PDA plates. Because the osmoregulation pathway is not known to regulate aerial hyphal growth and branching, in this study we further characterized the functions of the FgHog1 pathway in growth, pathogenesis, and development. The Fghog1, Fgpbs2, and Fgssk2 mutants were all reduced in growth rate, aerial hyphal growth, and hyphal branching angle. These mutants were not only hypersensitive to osmotic stress but also had increased sensitivity to oxidative, cytoplasm membrane, and cell wall stresses. The activation of FgHog1 was blocked in the Fgpbs2 and Fgssk2 mutants, indicating the sequential activation of FgSsk2-FgPbs2-FgHog1 cascade. Interestingly, the FgHog1 MAPK pathway mutants appeared to be sensitive to certain compounds present in PDA. They were female sterile but retained male fertility. We also used the metabolomics profiling approach to identify compatible solutes that were accumulated in the wild type but not in the Fghog1 deletion mutant. Overall, our results indicate that the FgSsk2-FgPbs2-FgHog1 MAPK cascade is important for regulating hyphal growth, branching, plant infection, and hyperosmotic and general stress responses in F. graminearum.
Conflict of interest statement
Figures





Similar articles
-
The AMT1 arginine methyltransferase gene is important for plant infection and normal hyphal growth in Fusarium graminearum.PLoS One. 2012;7(5):e38324. doi: 10.1371/journal.pone.0038324. Epub 2012 May 31. PLoS One. 2012. PMID: 22693618 Free PMC article.
-
The stress-activated protein kinase FgOS-2 is a key regulator in the life cycle of the cereal pathogen Fusarium graminearum.Mol Plant Microbe Interact. 2012 Sep;25(9):1142-56. doi: 10.1094/MPMI-02-12-0047-R. Mol Plant Microbe Interact. 2012. PMID: 22591226
-
Deletion of FgHOG1 Is Suppressive to the mgv1 Mutant by Stimulating Gpmk1 Activation and Avoiding Intracellular Turgor Elevation in Fusarium graminearum.Front Microbiol. 2019 May 22;10:1073. doi: 10.3389/fmicb.2019.01073. eCollection 2019. Front Microbiol. 2019. PMID: 31178834 Free PMC article.
-
The MAPKK FgMkk1 of Fusarium graminearum regulates vegetative differentiation, multiple stress response, and virulence via the cell wall integrity and high-osmolarity glycerol signaling pathways.Environ Microbiol. 2014 Jul;16(7):2023-37. doi: 10.1111/1462-2920.12334. Epub 2013 Dec 10. Environ Microbiol. 2014. PMID: 24237706
-
The cAMP-PKA pathway regulates growth, sexual and asexual differentiation, and pathogenesis in Fusarium graminearum.Mol Plant Microbe Interact. 2014 Jun;27(6):557-66. doi: 10.1094/MPMI-10-13-0306-R. Mol Plant Microbe Interact. 2014. PMID: 24450772
Cited by
-
A phosphorylated transcription factor regulates sterol biosynthesis in Fusarium graminearum.Nat Commun. 2019 Mar 15;10(1):1228. doi: 10.1038/s41467-019-09145-6. Nat Commun. 2019. PMID: 30874562 Free PMC article.
-
Epistatic Relationship between MGV1 and TRI6 in the Regulation of Biosynthetic Gene Clusters in Fusarium graminearum.J Fungi (Basel). 2023 Aug 2;9(8):816. doi: 10.3390/jof9080816. J Fungi (Basel). 2023. PMID: 37623587 Free PMC article.
-
Deciphering the Genomic Landscape and Virulence Mechanisms of the Wheat Powdery Mildew Pathogen Blumeria graminis f. sp. tritici Wtn1: Insights from Integrated Genome Assembly and Conidial Transcriptomics.J Fungi (Basel). 2024 Apr 3;10(4):267. doi: 10.3390/jof10040267. J Fungi (Basel). 2024. PMID: 38667938 Free PMC article.
-
R-SNARE FgSec22 is essential for growth, pathogenicity and DON production of Fusarium graminearum.Curr Genet. 2020 Apr;66(2):421-435. doi: 10.1007/s00294-019-01037-y. Epub 2019 Oct 30. Curr Genet. 2020. PMID: 31667538
-
The PKR regulatory subunit of protein kinase A (PKA) is involved in the regulation of growth, sexual and asexual development, and pathogenesis in Fusarium graminearum.Mol Plant Pathol. 2018 Apr;19(4):909-921. doi: 10.1111/mpp.12576. Epub 2017 Sep 20. Mol Plant Pathol. 2018. PMID: 28665481 Free PMC article.
References
-
- Goswami RS, Kistler HC (2004) Heading for disaster: Fusarium graminearum on cereal crops. Mol Plant Pathol 5: 515–525. - PubMed
-
- Bai GH, Shaner G (2004) Management and resistance in wheat and barley to Fusarium head blight. Annu Rev Phytopathol 42: 135–161. - PubMed
-
- Mesterhazy A (1995) Types and components of resistance to Fusarium head blight of wheat. Plant Breeding 114: 377–386.
-
- Rudd JC, Horsley RD, McKendry AL, Elias EM (2001) Host plant resistance genes for Fusarium head blight: Sources, mechanisms, and utility in conventional breeding systems. Crop Sci 41: 620–627.
-
- Saito H, Tatebayashi K (2004) Regulation of the osmoregulatory HOG MAPK cascade in yeast. J Biochem 136: 267–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

