The ability to enhance the solubility of its fusion partners is an intrinsic property of maltose-binding protein but their folding is either spontaneous or chaperone-mediated
- PMID: 23166722
- PMCID: PMC3500312
- DOI: 10.1371/journal.pone.0049589
The ability to enhance the solubility of its fusion partners is an intrinsic property of maltose-binding protein but their folding is either spontaneous or chaperone-mediated
Abstract
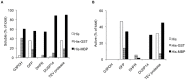
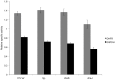
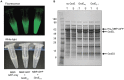
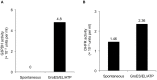
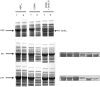
Escherichia coli maltose binding protein (MBP) is commonly used to promote the solubility of its fusion partners. To investigate the mechanism of solubility enhancement by MBP, we compared the properties of MBP fusion proteins refolded in vitro with those of the corresponding fusion proteins purified under native conditions. We fused five aggregation-prone passenger proteins to 3 different N-terminal tags: His₆-MBP, His₆-GST and His₆. After purifying the 15 fusion proteins under denaturing conditions and refolding them by rapid dilution, we recovered far more of the soluble MBP fusion proteins than their GST- or His-tagged counterparts. Hence, we can reproduce the solubilizing activity of MBP in a simple in vitro system, indicating that no additional factors are required to mediate this effect. We assayed both the soluble fusion proteins and their TEV protease digestion products (i.e., with the N-terminal tag removed) for biological activity. Little or no activity was detected for some fusion proteins whereas others were quite active. When the MBP fusions proteins were purified from E. coli under native conditions they were all substantially active. These results indicate that the ability of MBP to promote the solubility of its fusion partners in vitro sometimes, but not always, results in their proper folding. We show that the folding of some passenger proteins is mediated by endogenous chaperones in vivo. Hence, MBP serves as a passive participant in the folding process; passenger proteins either fold spontaneously or with the assistance of chaperones.
Conflict of interest statement
Figures

Similar articles
-
Positional effects of fusion partners on the yield and solubility of MBP fusion proteins.Protein Expr Purif. 2015 Jun;110:159-64. doi: 10.1016/j.pep.2015.03.004. Epub 2015 Mar 14. Protein Expr Purif. 2015. PMID: 25782741 Free PMC article.
-
Enhancing the solubility of recombinant proteins in Escherichia coli by using hexahistidine-tagged maltose-binding protein as a fusion partner.Methods Mol Biol. 2011;705:259-74. doi: 10.1007/978-1-61737-967-3_16. Methods Mol Biol. 2011. PMID: 21125392 Review.
-
Single amino acid substitutions on the surface of Escherichia coli maltose-binding protein can have a profound impact on the solubility of fusion proteins.Protein Sci. 2001 Mar;10(3):622-30. doi: 10.1110/ps.45201. Protein Sci. 2001. PMID: 11344330 Free PMC article.
-
Unrelated solubility-enhancing fusion partners MBP and NusA utilize a similar mode of action.Biotechnol Bioeng. 2014 Dec;111(12):2407-11. doi: 10.1002/bit.25317. Epub 2014 Aug 25. Biotechnol Bioeng. 2014. PMID: 24942647 Free PMC article.
-
The remarkable solubility-enhancing power of Escherichia coli maltose-binding protein.Postepy Biochem. 2016;62(3):377-382. Postepy Biochem. 2016. PMID: 28132493 Review. English.
Cited by
-
Dual targeting of the chemokine receptors CXCR4 and ACKR3 with novel engineered chemokines.J Biol Chem. 2015 Sep 11;290(37):22385-97. doi: 10.1074/jbc.M115.675108. Epub 2015 Jul 27. J Biol Chem. 2015. PMID: 26216880 Free PMC article.
-
A fusion protein designed for soluble expression, rapid purification, and enhanced stability of parasporin-2 with potential therapeutic applications.Biotechnol Rep (Amst). 2024 Aug 5;43:e00851. doi: 10.1016/j.btre.2024.e00851. eCollection 2024 Sep. Biotechnol Rep (Amst). 2024. PMID: 39219730 Free PMC article.
-
A new fusion protein platform for quantitatively measuring activity of multiple proteases.Microb Cell Fact. 2014 Mar 21;13(1):44. doi: 10.1186/1475-2859-13-44. Microb Cell Fact. 2014. PMID: 24649897 Free PMC article.
-
Expression and in vitro functional analyses of recombinant Gam1 protein.Protein Expr Purif. 2015 Jan;105:47-53. doi: 10.1016/j.pep.2014.10.005. Epub 2014 Oct 16. Protein Expr Purif. 2015. PMID: 25450237 Free PMC article.
-
Maltose binding protein-fusion enhances the bioactivity of truncated forms of pig myostatin propeptide produced in E. coli.PLoS One. 2017 Apr 3;12(4):e0174956. doi: 10.1371/journal.pone.0174956. eCollection 2017. PLoS One. 2017. PMID: 28369115 Free PMC article.
References
-
- Waugh DS (2005) Making the most of affinity tags. Trends Biotechnol 23: 316–320. - PubMed
-
- Sun P, Tropea JE, Waugh DS (2011) Enhancing the solubility of recombinant proteins in Escherichia coli by using hexahistidine-tagged maltose-binding protein as a fusion partner. Methods Mol Biol 705: 259–274. - PubMed
-
- Zhang YB, Howitt J, McCorkle S, Lawrence P, Springer K, et al. (2004) Protein aggregation during overexpression limited by peptide extensions with large net negative charge. Protein Expr Purif 36: 207–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

