Bidirectional scaling of astrocytic metabotropic glutamate receptor signaling following long-term changes in neuronal firing rates
- PMID: 23166735
- PMCID: PMC3499417
- DOI: 10.1371/journal.pone.0049637
Bidirectional scaling of astrocytic metabotropic glutamate receptor signaling following long-term changes in neuronal firing rates
Abstract
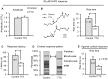
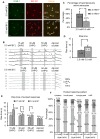
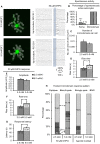
Very little is known about the ability of astrocytic receptors to exhibit plasticity as a result of changes in neuronal activity. Here we provide evidence for bidirectional scaling of astrocytic group I metabotropic glutamate receptor signaling in acute mouse hippocampal slices following long-term changes in neuronal firing rates. Plasticity of astrocytic mGluRs was measured by recording spontaneous and evoked Ca²⁺ elevations in both astrocytic somata and processes. An exogenous astrocytic Gq G protein-coupled receptor was resistant to scaling, suggesting that the alterations in astrocyte Ca²⁺ signaling result from changes in activity of the surface mGluRs rather than a change in intracellular G protein signaling molecules. These findings suggest that astrocytes actively detect shifts in neuronal firing rates and adjust their receptor signaling accordingly. This type of long-term plasticity in astrocytes resembles neuronal homeostatic plasticity and might be important to ensure an optimal or expected level of input from neurons.
Conflict of interest statement
Figures







Similar articles
-
Inducing plasticity of astrocytic receptors by manipulation of neuronal firing rates.J Vis Exp. 2014 Mar 20;(85):51458. doi: 10.3791/51458. J Vis Exp. 2014. PMID: 24686723 Free PMC article.
-
Astrocytic group I mGluR-dependent potentiation of astrocytic glutamate and potassium uptake.J Neurophysiol. 2013 May;109(9):2404-14. doi: 10.1152/jn.00517.2012. Epub 2013 Feb 20. J Neurophysiol. 2013. PMID: 23427307
-
Astrocytes in 17beta-estradiol treated mixed hippocampal cultures show attenuated calcium response to neuronal activity.Glia. 2006 Jun;53(8):817-26. doi: 10.1002/glia.20341. Glia. 2006. PMID: 16565986
-
Local energy on demand: Are 'spontaneous' astrocytic Ca2+-microdomains the regulatory unit for astrocyte-neuron metabolic cooperation?Brain Res Bull. 2018 Jan;136:54-64. doi: 10.1016/j.brainresbull.2017.04.011. Epub 2017 Apr 24. Brain Res Bull. 2018. PMID: 28450076 Review.
-
Control of the phosphorylation of the astrocyte marker glial fibrillary acidic protein (GFAP) in the immature rat hippocampus by glutamate and calcium ions: possible key factor in astrocytic plasticity.Braz J Med Biol Res. 1997 Mar;30(3):325-38. doi: 10.1590/s0100-879x1997000300005. Braz J Med Biol Res. 1997. PMID: 9246230 Review.
Cited by
-
Astrocyte-Selective Volume Increase in Elevated Extracellular Potassium Conditions Is Mediated by the Na+/K+ ATPase and Occurs Independently of Aquaporin 4.ASN Neuro. 2020 Jan-Dec;12:1759091420967152. doi: 10.1177/1759091420967152. ASN Neuro. 2020. PMID: 33092407 Free PMC article.
-
Astrocyte and Neuronal Plasticity in the Somatosensory System.Neural Plast. 2015;2015:732014. doi: 10.1155/2015/732014. Epub 2015 Aug 4. Neural Plast. 2015. PMID: 26345481 Free PMC article. Review.
-
Diversity of Evoked Astrocyte Ca2+ Dynamics Quantified through Experimental Measurements and Mathematical Modeling.Front Syst Neurosci. 2017 Oct 23;11:79. doi: 10.3389/fnsys.2017.00079. eCollection 2017. Front Syst Neurosci. 2017. PMID: 29109680 Free PMC article.
-
A hierarchy of manganese competition and entry in organotypic hippocampal slice cultures.NMR Biomed. 2021 Apr;34(4):e4476. doi: 10.1002/nbm.4476. Epub 2021 Feb 3. NMR Biomed. 2021. PMID: 33538073 Free PMC article.
-
Inducing plasticity of astrocytic receptors by manipulation of neuronal firing rates.J Vis Exp. 2014 Mar 20;(85):51458. doi: 10.3791/51458. J Vis Exp. 2014. PMID: 24686723 Free PMC article.
References
-
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, et al. (2006) Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci 9: 816–823. - PubMed
-
- Schummers J, Yu H, Sur M (2008) Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320: 1638–1643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

