Evidence of Bacteroides fragilis protection from Bartonella henselae-induced damage
- PMID: 23166739
- PMCID: PMC3499472
- DOI: 10.1371/journal.pone.0049653
Evidence of Bacteroides fragilis protection from Bartonella henselae-induced damage
Abstract
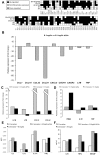
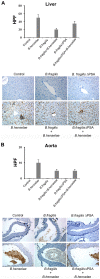
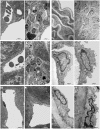
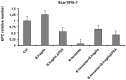
Bartonella henselae is able to internalize endothelial progenitor cells (EPCs), which are resistant to the infection of other common pathogens. Bacteroides fragilis is a gram-negative anaerobe belonging to the gut microflora. It protects from experimental colitis induced by Helicobacter hepaticus through the polysaccharide A (PSA). The aim of our study was to establish: 1) whether B. fragilis colonization could protect from B. henselae infection; if this event may have beneficial effects on EPCs, vascular system and tissues. Our in vitro results establish for the first time that B. fragilis can internalize EPCs and competes with B. henselae during coinfection. We observed a marked activation of the inflammatory response by Real-time PCR and ELISA in coinfected cells compared to B. henselae-infected cells (63 vs 23 up-regulated genes), and after EPCs infection with mutant B. fragilis ΔPSA (≅90% up-regulated genes) compared to B. fragilis. Interestingly, in a mouse model of coinfection, morphological and ultrastructural analyses by hematoxylin-eosin staining and electron microscopy on murine tissues revealed that damages induced by B. henselae can be prevented in the coinfection with B. fragilis but not with its mutant B. fragilis ΔPSA. Moreover, immunohistochemistry analysis with anti-Bartonella showed that the number of positive cells per field decreased of at least 50% in the liver (20±4 vs 50±8), aorta (5±1 vs 10±2) and spleen (25±3 vs 40±6) sections of mice coinfected compared to mice infected only with B. henselae. This decrease was less evident in the coinfection with ΔPSA strain (35±6 in the liver, 5±1 in the aorta and 30±5 in the spleen). Finally, B. fragilis colonization was also able to restore the EPC decrease observed in mice infected with B. henselae (0.65 vs 0.06 media). Thus, our data establish that B. fragilis colonization is able to prevent B. henselae damages through PSA.
Conflict of interest statement
Figures



Similar articles
-
Novel Approach for Evaluation of Bacteroides fragilis Protective Role against Bartonella henselae Liver Damage in Immunocompromised Murine Model.Front Microbiol. 2016 Nov 7;7:1750. doi: 10.3389/fmicb.2016.01750. eCollection 2016. Front Microbiol. 2016. PMID: 27872616 Free PMC article.
-
Bartonella henselae Persistence within Mesenchymal Stromal Cells Enhances Endothelial Cell Activation and Infectibility That Amplifies the Angiogenic Process.Infect Immun. 2021 Jul 15;89(8):e0014121. doi: 10.1128/IAI.00141-21. Epub 2021 Jul 15. Infect Immun. 2021. PMID: 34031126 Free PMC article.
-
Differential effects of Bartonella henselae on human and feline macro- and micro-vascular endothelial cells.PLoS One. 2011;6(5):e20204. doi: 10.1371/journal.pone.0020204. Epub 2011 May 27. PLoS One. 2011. PMID: 21637717 Free PMC article.
-
Bacillary angiomatosis in immunocompromised patients.AIDS. 1998 Oct 1;12(14):1793-803. doi: 10.1097/00002030-199814000-00011. AIDS. 1998. PMID: 9792380 Review.
-
The Intestinal Commensal, Bacteroides fragilis, Modulates Host Responses to Viral Infection and Therapy: Lessons for Exploration during Mycobacterium tuberculosis Infection.Infect Immun. 2022 Jan 25;90(1):e0032121. doi: 10.1128/IAI.00321-21. Epub 2021 Oct 4. Infect Immun. 2022. PMID: 34606367 Free PMC article. Review.
Cited by
-
Commensal Obligate Anaerobic Bacteria and Health: Production, Storage, and Delivery Strategies.Front Bioeng Biotechnol. 2020 Jun 5;8:550. doi: 10.3389/fbioe.2020.00550. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32582673 Free PMC article. Review.
-
In vitro Synergy of Polyphenolic Extracts From Honey, Myrtle and Pomegranate Against Oral Pathogens, S. mutans and R. dentocariosa.Front Microbiol. 2020 Jul 24;11:1465. doi: 10.3389/fmicb.2020.01465. eCollection 2020. Front Microbiol. 2020. PMID: 32849317 Free PMC article.
-
Gut Bacteroides species in health and disease.Gut Microbes. 2021 Jan-Dec;13(1):1-20. doi: 10.1080/19490976.2020.1848158. Gut Microbes. 2021. PMID: 33535896 Free PMC article. Review.
-
Transient Anomalous Diffusion MRI in Excised Mouse Spinal Cord: Comparison Among Different Diffusion Metrics and Validation With Histology.Front Neurosci. 2022 Feb 15;15:797642. doi: 10.3389/fnins.2021.797642. eCollection 2021. Front Neurosci. 2022. PMID: 35242002 Free PMC article.
-
Bacteroides ovatus Promotes IL-22 Production and Reduces Trinitrobenzene Sulfonic Acid-Driven Colonic Inflammation.Am J Pathol. 2021 Apr;191(4):704-719. doi: 10.1016/j.ajpath.2021.01.009. Epub 2021 Jan 28. Am J Pathol. 2021. PMID: 33516788 Free PMC article.
References
-
- Dehio C, Berry C, Bartenschlager R (2012) Persistent intracellular pathogens. FEMS Microbiol Rev 36: 513. - PubMed
-
- Pulliainen AT, Dehio C (2012) Persistence of Bartonella spp. stealth pathogens: from subclinical infections to vasoproliferative tumor formation. FEMS Microbiol Rev 36: 563–599. - PubMed
-
- Eicher SC, Dehio C (2012) Bartonella entry mechanisms into mammalian host cells. Cell Microbiol. Apr 23. [Epub ahead of print]. - PubMed
-
- Maguiña C, Guerra H, Ventosilla P (2009) Bartonellosis. Clin Dermatol 27: 271–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

