Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses
- PMID: 23166749
- PMCID: PMC3498223
- DOI: 10.1371/journal.pone.0049701
Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses
Abstract
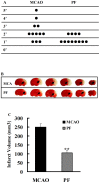
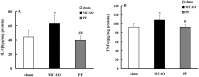
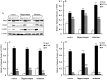
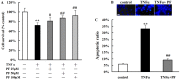
Paeoniflorin (PF), the principal component of Paeoniae Radix prescribed in traditional Chinese medicine, has been reported to exhibit many pharmacological effects including protection against ischemic injury. However, the mechanisms underlying the protective effects of PF on cerebral ischemia are still under investigation. The present study showed that PF treatment for 14 days could significantly inhibit transient middle cerebral artery occlusion (MCAO)-induced over-activation of astrocytes and microglia, and prevented up-regulations of pro-inflamamtory mediators (TNFα, IL-1β, iNOS, COX(2) and 5-LOX) in plasma and brain. Further study demonstrated that chronic treatment with PF suppressed the activations of JNK and p38 MAPK, but enhanced ERK activation. And PF could reverse ischemia-induced activation of NF-κB signaling pathway. Moreover, our in vitro study revealed that PF treatment protected against TNFα-induced cell apoptosis and neuronal loss. Taken together, the present study demonstrates that PF produces a delayed protection in the ischemia-injured rats via inhibiting MAPKs/NF-κB mediated peripheral and cerebral inflammatory response. Our study reveals that PF might be a potential neuroprotective agent for stroke.
Conflict of interest statement
Figures




References
-
- Girouard H, Iadecola C (2006) Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100 1: 328–335. - PubMed
-
- Mander P, Borutaite V, Moncada S, Brown GC (2005) Nitric oxide from inflammatory-activated glia synergizes with hypoxia to induce neuronal death. J Neurosci Res 79 1–2: 208–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

