IFN regulatory factor 8 is a key constitutive determinant of the morphological and molecular properties of microglia in the CNS
- PMID: 23166780
- PMCID: PMC3498170
- DOI: 10.1371/journal.pone.0049851
IFN regulatory factor 8 is a key constitutive determinant of the morphological and molecular properties of microglia in the CNS
Erratum in
- PLoS One. 2013;8(5). doi:10.1371/annotation/492fdf80-c999-4947-b569-96af8cb4e9d9
Abstract
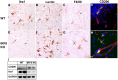
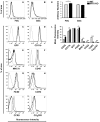
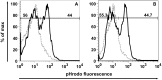
IFN regulatory factor (IRF) 8 is a transcription factor that has a key role in the cellular response to IFN-γ and is pivotal in myeloid cell differentiation. Whether IRF8 plays a role in the development and function of microglia, the tissue-resident myeloid cells of the brain, is unknown. Here, we show IRF8 is a constitutively produced nuclear factor in microglia, which suggested that IRF8 might also be a key homeostatic transcriptional determinant of the microglial cell phenotype. In support of this, in mice with a targeted disruption of the IRF8 gene, microglia were increased in number and showed gross alterations in morphology and surface area. In situ analysis of some key myeloid markers revealed that IRF8-deficient microglia had significantly reduced levels of Iba1, but increased levels of CD206 (mannose receptor) and F4/80 as well as increased tomato lectin binding. Analysis of microglia ex vivo revealed IRF8-deficient microglia had significantly increased levels of CD45, CD11b and F4/80, but significantly decreased levels of the chemokine receptors CCR2, CCR5 and CX3CR1. The known involvement of some of these molecular markers in membrane dynamics and phagocytosis led us to examine the phagocytic capacity of cultured IRF8-deficient microglia, however, this was found to be similar to wild type microglia. We conclude IRF8 is a constitutively produced nuclear factor in resident microglia of the CNS being a crucial transcriptional determinant of the phenotype of these cells in the healthy brain.
Conflict of interest statement
Figures



References
-
- Prinz M, Mildner A (2010) Microglia in the CNS: Immigrants from another world. Glia 59: 177–187. - PubMed
-
- Ransohoff RM, Cardona AE (2010) The myeloid cells of the central nervous system parenchyma. Nature 468: 253–262. - PubMed
-
- Taniguchi T, Ogasawara K, Takaoka A, Tanaka N (2001) IRF family of transcription factors as regulators of host defense. Ann Rev Immunol 19: 623–655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

