A simplified method for the efficient refolding and purification of recombinant human GM-CSF
- PMID: 23166789
- PMCID: PMC3498172
- DOI: 10.1371/journal.pone.0049891
A simplified method for the efficient refolding and purification of recombinant human GM-CSF
Abstract
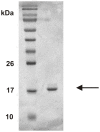
Human granulocyte macrophage colony-stimulating factor (hGM-CSF) is a haematopoietic growth factor and proinflammatory cytokine. Recombinant hGM-CSF is important not only as a research tool but also as a biotherapeutic. However, rhGM-CSF expressed in E. coli is known to form inclusion bodies of misfolded, aggregated protein. Refolding and subsequent purification of rhGM-CSF from inclusion bodies is difficult with low yields of bioactive protein being produced. Here we describe a method for the isolation, refolding and purification of bioactive rhGM-CSF from inclusion bodies. The method is straightforward, not requiring extensive experience in protein refolding nor purification and using standard laboratory equipment.
Conflict of interest statement
Figures

Similar articles
-
Purification of recombinant human granulocyte-macrophage colony stimulating factor expressed in Escherichia coli.Chin J Biotechnol. 1995;11(3):157-62. Chin J Biotechnol. 1995. PMID: 8679931
-
Optimization of human granulocyte macrophage-colony stimulating factor (hGM-CSF) expression using asparaginase and xylanase gene's signal sequences in Escherichia coli.Appl Biochem Biotechnol. 2011 Sep;165(2):523-37. doi: 10.1007/s12010-011-9272-5. Epub 2011 May 12. Appl Biochem Biotechnol. 2011. PMID: 21562804
-
Purification of recombinant human granulocyte-macrophage colony-stimulating factor from the inclusion bodies produced by transformed Escherichia coli cells.J Chromatogr A. 1994 Sep 9;679(1):67-83. doi: 10.1016/0021-9673(94)80312-9. J Chromatogr A. 1994. PMID: 7951992
-
Expression, Solubilization, Refolding and Final Purification of Recombinant Proteins as Expressed in the form of "Classical Inclusion Bodies" in E. coli.Protein Pept Lett. 2021;28(2):122-130. doi: 10.2174/0929866527999200729182831. Protein Pept Lett. 2021. PMID: 32729411 Review.
-
Solubilization and refolding of bacterial inclusion body proteins.J Biosci Bioeng. 2005 Apr;99(4):303-10. doi: 10.1263/jbb.99.303. J Biosci Bioeng. 2005. PMID: 16233795 Review.
Cited by
-
Soluble Prokaryotic Overexpression and Purification of Human GM-CSF Using the Protein Disulfide Isomerase b'a' Domain.Int J Mol Sci. 2021 May 17;22(10):5267. doi: 10.3390/ijms22105267. Int J Mol Sci. 2021. PMID: 34067755 Free PMC article.
-
Effective refolding of a cysteine rich glycoside hydrolase family 19 recombinant chitinase from Streptomyces griseus by reverse dilution and affinity chromatography.PLoS One. 2020 Oct 22;15(10):e0241074. doi: 10.1371/journal.pone.0241074. eCollection 2020. PLoS One. 2020. PMID: 33091044 Free PMC article.
-
Serodiagnostic evaluation of recombinant CdtB of S. Typhi as a potential candidate for acute typhoid.Immunol Res. 2018 Aug;66(4):503-512. doi: 10.1007/s12026-018-9009-4. Immunol Res. 2018. PMID: 29931558
-
Characterization of a novel + 70 Da modification in rhGM-CSF expressed in E. coli using chemical assays in combination with mass spectrometry.Amino Acids. 2022 Apr;54(4):601-613. doi: 10.1007/s00726-021-03004-9. Epub 2021 Aug 28. Amino Acids. 2022. PMID: 34453584 Free PMC article.
-
Cytokine refacing effect reduces granulocyte macrophage colony-stimulating factor susceptibility to antibody neutralization.Protein Eng Des Sel. 2015 Oct;28(10):461-6. doi: 10.1093/protein/gzv019. Epub 2015 Apr 7. Protein Eng Des Sel. 2015. PMID: 25855658 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

