Function annotation of hepatic retinoid x receptor α based on genome-wide DNA binding and transcriptome profiling
- PMID: 23166811
- PMCID: PMC3499475
- DOI: 10.1371/journal.pone.0050013
Function annotation of hepatic retinoid x receptor α based on genome-wide DNA binding and transcriptome profiling
Abstract
Background: Retinoid x receptor α (RXRα) is abundantly expressed in the liver and is essential for the function of other nuclear receptors. Using chromatin immunoprecipitation sequencing and mRNA profiling data generated from wild type and RXRα-null mouse livers, the current study identifies the bona-fide hepatic RXRα targets and biological pathways. In addition, based on binding and motif analysis, the molecular mechanism by which RXRα regulates hepatic genes is elucidated in a high-throughput manner.
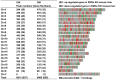
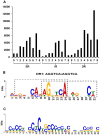
Principal findings: Close to 80% of hepatic expressed genes were bound by RXRα, while 16% were expressed in an RXRα-dependent manner. Motif analysis predicted direct repeat with a spacer of one nucleotide as the most prevalent RXRα binding site. Many of the 500 strongest binding motifs overlapped with the binding motif of specific protein 1. Biological functional analysis of RXRα-dependent genes revealed that hepatic RXRα deficiency mainly resulted in up-regulation of steroid and cholesterol biosynthesis-related genes and down-regulation of translation- as well as anti-apoptosis-related genes. Furthermore, RXRα bound to many genes that encode nuclear receptors and their cofactors suggesting the central role of RXRα in regulating nuclear receptor-mediated pathways.
Conclusions: This study establishes the relationship between RXRα DNA binding and hepatic gene expression. RXRα binds extensively to the mouse genome. However, DNA binding does not necessarily affect the basal mRNA level. In addition to metabolism, RXRα dictates the expression of genes that regulate RNA processing, translation, and protein folding illustrating the novel roles of hepatic RXRα in post-transcriptional regulation.
Conflict of interest statement
Figures



References
-
- De Luca LM (1991) Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J 5: 2924–2933. - PubMed
-
- Shulman AI, Mangelsdorf DJ (2005) Retinoid x receptor heterodimers in the metabolic syndrome. N Engl J Med 353: 604–615. - PubMed
-
- Lawrence JA, Merino MJ, Simpson JF, Manrow RE, Page DL, et al. (1998) A high-risk lesion for invasive breast cancer, ductal carcinoma in situ, exhibits frequent overexpression of retinoid X receptor. Cancer Epidemiol Biomarkers Prev 7: 29–35. - PubMed
-
- Qu L, Tang X (2010) Bexarotene: a promising anticancer agent. Cancer Chemother Pharmacol 65: 201–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

