Early metabolic defects in dexamethasone-exposed and undernourished intrauterine growth restricted rats
- PMID: 23166830
- PMCID: PMC3500352
- DOI: 10.1371/journal.pone.0050131
Early metabolic defects in dexamethasone-exposed and undernourished intrauterine growth restricted rats
Abstract
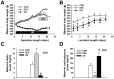
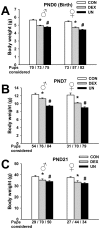
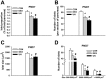
Poor fetal growth, also known as intrauterine growth restriction (IUGR), is a worldwide health concern. IUGR is commonly associated with both an increased risk in perinatal mortality and a higher prevalence of developing chronic metabolic diseases later in life. Obesity, type 2 diabetes or metabolic syndrome could result from noxious "metabolic programming." In order to better understand early alterations involved in metabolic programming, we modeled IUGR rat pups through either prenatal exposure to synthetic glucocorticoid (dams infused with dexamethasone 100 µg/kg/day, DEX) or prenatal undernutrition (dams feeding restricted to 30% of ad libitum intake, UN). Physiological (glucose and insulin tolerance), morphometric (automated tissue image analysis) and transcriptomic (quantitative PCR) approaches were combined during early life of these IUGR pups with a special focus on their endocrine pancreas and adipose tissue development. In the absence of catch-up growth before weaning, DEX and UN IUGR pups both presented basal hyperglycaemia, decreased glucose tolerance, and pancreatic islet atrophy. Other early metabolic defects were model-specific: DEX pups presented decreased insulin sensitivity whereas UN pups exhibited lowered glucose-induced insulin secretion and more marked alterations in gene expression of pancreatic islet and adipose tissue development regulators. In conclusion, these results show that before any catch-up growth, IUGR rats present early physiologic, morphologic and transcriptomic defects, which can be considered as initial mechanistic basis of metabolic programming.
Conflict of interest statement
Figures



References
-
- Vuguin P, Raab E, Liu B, Barzilai N, Simmons R (2004) Hepatic insulin resistance precedes the development of diabetes in a model of intrauterine growth retardation. Diabetes 53: 2617–2622. - PubMed
-
- Tapanainen PJ, Bang P, Wilson K, Unterman TG, Vreman HJ, et al. (1994) Maternal hypoxia as a model for intrauterine growth retardation: effects on insulin-like growth factors and their binding proteins. Pediatr Res 36: 152–158. - PubMed
-
- Eriksson UJ, Jansson L (1984) Diabetes in pregnancy: decreased placental blood flow and disturbed fetal development in the rat. Pediatr Res 18: 735–738. - PubMed
-
- Jimenez-Chillaron JC, Hernandez-Valencia M, Reamer C, Fisher S, Joszi A, et al. (2005) Beta-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes: a murine model. Diabetes 54: 702–711. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

