Sensitivity to lysosome-dependent cell death is directly regulated by lysosomal cholesterol content
- PMID: 23166840
- PMCID: PMC3500374
- DOI: 10.1371/journal.pone.0050262
Sensitivity to lysosome-dependent cell death is directly regulated by lysosomal cholesterol content
Abstract
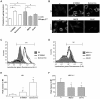
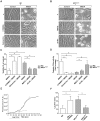
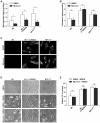
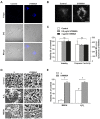
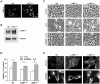
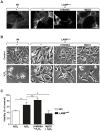
Alterations in lipid homeostasis are implicated in several neurodegenerative diseases, although the mechanisms responsible are poorly understood. We evaluated the impact of cholesterol accumulation, induced by U18666A, quinacrine or mutations in the cholesterol transporting Niemann-Pick disease type C1 (NPC1) protein, on lysosomal stability and sensitivity to lysosome-mediated cell death. We found that neurons with lysosomal cholesterol accumulation were protected from oxidative stress-induced apoptosis. In addition, human fibroblasts with cholesterol-loaded lysosomes showed higher lysosomal membrane stability than controls. Previous studies have shown that cholesterol accumulation is accompanied by the storage of lipids such as sphingomyelin, glycosphingolipids and sphingosine and an up regulation of lysosomal associated membrane protein-2 (LAMP-2), which may also influence lysosomal stability. However, in this study the use of myriocin and LAMP deficient fibroblasts excluded these factors as responsible for the rescuing effect and instead suggested that primarily lysosomal cholesterol content determineD the cellular sensitivity to toxic insults. Further strengthening this concept, depletion of cholesterol using methyl-β-cyclodextrin or 25-hydroxycholesterol decreased the stability of lysosomes and cells became more prone to undergo apoptosis. In conclusion, cholesterol content regulated lysosomal membrane permeabilization and thereby influenced cell death sensitivity. Our data suggests that lysosomal cholesterol modulation might be used as a therapeutic strategy for conditions associated with accelerated or repressed apoptosis.
Conflict of interest statement
Figures
References
-
- Repnik U, Stoka V, Turk V, Turk B (2012) Lysosomes and lysosomal cathepsins in cell death. Biochim Biophys Acta 1824: 22–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

