Nonassociative plasticity alters competitive interactions among mixture components in early olfactory processing
- PMID: 23167675
- PMCID: PMC3538925
- DOI: 10.1111/ejn.12021
Nonassociative plasticity alters competitive interactions among mixture components in early olfactory processing
Abstract
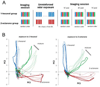
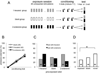
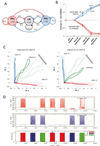
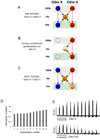
Experience-related plasticity is an essential component of networks involved in early olfactory processing. However, the mechanisms and functions of plasticity in these neural networks are not well understood. We studied nonassociative plasticity by evaluating responses to two pure odors (A and X) and their binary mixture using calcium imaging of odor-elicited activity in output neurons of the honey bee antennal lobe. Unreinforced exposure to A or X produced no change in the neural response elicited by the pure odors. However, exposure to one odor (e.g. A) caused the response to the mixture to become more similar to that of the other component (X). We also show in behavioral analyses that unreinforced exposure to A caused the mixture to become perceptually more similar to X. These results suggest that nonassociative plasticity modifies neural networks in such a way that it affects local competitive interactions among mixture components. We used a computational model to evaluate the most likely targets for modification. Hebbian modification of synapses from inhibitory local interneurons to projection neurons most reliably produced the observed shift in response to the mixture. These results are consistent with a model in which the antennal lobe acts to filter olfactory information according to its relevance for performing a particular task.
© 2012 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Conflict of interest statement
None of the authors have a conflict of interest for this work.
Figures



Similar articles
-
Learning modifies odor mixture processing to improve detection of relevant components.J Neurosci. 2015 Jan 7;35(1):179-97. doi: 10.1523/JNEUROSCI.2345-14.2015. J Neurosci. 2015. PMID: 25568113 Free PMC article.
-
Ensemble response in mushroom body output neurons of the honey bee outpaces spatiotemporal odor processing two synapses earlier in the antennal lobe.PLoS One. 2012;7(11):e50322. doi: 10.1371/journal.pone.0050322. Epub 2012 Nov 29. PLoS One. 2012. PMID: 23209711 Free PMC article.
-
Plasticity in inhibitory networks improves pattern separation in early olfactory processing.Commun Biol. 2025 Apr 9;8(1):590. doi: 10.1038/s42003-025-07879-2. Commun Biol. 2025. PMID: 40204909 Free PMC article.
-
Odor processing in the cockroach antennal lobe-the network components.Cell Tissue Res. 2021 Jan;383(1):59-73. doi: 10.1007/s00441-020-03387-3. Epub 2021 Jan 23. Cell Tissue Res. 2021. PMID: 33486607 Free PMC article. Review.
-
Learning-dependent plasticity in the antennal lobe improves discrimination and recognition of odors in the honeybee.Cell Tissue Res. 2021 Jan;383(1):165-175. doi: 10.1007/s00441-020-03396-2. Epub 2021 Jan 29. Cell Tissue Res. 2021. PMID: 33511470 Review.
Cited by
-
Simultaneous long-term recordings at two neuronal processing stages in behaving honeybees.J Vis Exp. 2014 Jul 21;(89):51750. doi: 10.3791/51750. J Vis Exp. 2014. PMID: 25080029 Free PMC article.
-
Apis mellifera octopamine receptor 1 (AmOA1) expression in antennal lobe networks of the honey bee (Apis mellifera) and fruit fly (Drosophila melanogaster).Front Syst Neurosci. 2013 Oct 25;7:70. doi: 10.3389/fnsys.2013.00070. eCollection 2013. Front Syst Neurosci. 2013. PMID: 24187534 Free PMC article.
-
Olfactory coding in the insect brain: data and conjectures.Eur J Neurosci. 2014 Jun;39(11):1784-95. doi: 10.1111/ejn.12558. Epub 2014 Apr 3. Eur J Neurosci. 2014. PMID: 24698302 Free PMC article. Review.
-
Granger Causality Analysis of Transient Calcium Dynamics in the Honey Bee Antennal Lobe Network.Insects. 2023 Jun 9;14(6):539. doi: 10.3390/insects14060539. Insects. 2023. PMID: 37367355 Free PMC article.
-
Octopamine modulates activity of neural networks in the honey bee antennal lobe.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013 Nov;199(11):947-62. doi: 10.1007/s00359-013-0805-y. Epub 2013 May 17. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013. PMID: 23681219 Free PMC article.
References
-
- Abel R, Rybak J, Menzel R. Structure and response patterns of olfactory interneurons in the honeybee, Apis mellifera. J. Comp. Neurol. 2001;437:363–383. - PubMed
-
- Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. - PubMed
-
- Bitterman ME, Menzel R, Fietz A, Schafer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) J. Comp. Psychol. 1983;97:107–119. - PubMed
-
- Chandra SB, Wright GA, Smith BH. Latent inhibition in the honey bee, Apis mellifera: Is it a unitary phenomenon? Anim. Cogn. 2010;13:805–815. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

