An oxidopyrylium cyclization/ring-opening route to polysubstituted α-hydroxytropolones
- PMID: 23167954
- PMCID: PMC3518617
- DOI: 10.1021/ol302892g
An oxidopyrylium cyclization/ring-opening route to polysubstituted α-hydroxytropolones
Abstract
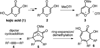
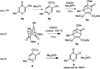
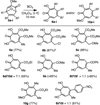
α-Hydroxytropolones are a class of molecules with therapeutic potential against several human diseases. However, structure-activity relationship studies on these molecules have been limited due to a scarcity of efficient synthetic methods to access them. It is demonstrated herein that α-hydroxytropolones can be generated through a BCl(3)-mediated ring-opening/aromatization/demethylation process on 8-oxabicyclo[3.2.1]octenes. Used in conjunction with an improved method based on established oxidopyrylium dipolar cycloadditions, several polysubstituted α-hydroxytropolones can be accessed in three steps from readily available α-hydroxy-γ-pyrones.
Figures



Similar articles
-
Discovery and Development of a Three-Component Oxidopyrylium [5 + 2] Cycloaddition.J Org Chem. 2016 May 6;81(9):3744-51. doi: 10.1021/acs.joc.6b00394. Epub 2016 Apr 8. J Org Chem. 2016. PMID: 27018974 Free PMC article.
-
Fluorous-Phase Approach to α-Hydroxytropolone Synthesis.J Org Chem. 2018 Feb 2;83(3):1478-1485. doi: 10.1021/acs.joc.7b03032. Epub 2018 Jan 22. J Org Chem. 2018. PMID: 29338231 Free PMC article.
-
Ring expansion of sulfur substituted p-quinamines: regiospecific synthesis of 4-aminotropones.Chem Commun (Camb). 2005 Feb 28;(8):1007-9. doi: 10.1039/b414666b. Epub 2005 Jan 5. Chem Commun (Camb). 2005. PMID: 15719098
-
The discovery of a novel route to highly substituted α-tropolones enables expedient entry to the core of the gukulenins.Org Lett. 2015 Apr 17;17(8):2030-3. doi: 10.1021/acs.orglett.5b00841. Epub 2015 Apr 3. Org Lett. 2015. PMID: 25839211
-
Diels-Alder Additions as Mechanistic Probes-Interception of Silyl-Isoindenes: Organometallic Derivatives of Polyphenylated Cycloheptatrienes and Related Seven-Membered Rings.Molecules. 2020 Oct 15;25(20):4730. doi: 10.3390/molecules25204730. Molecules. 2020. PMID: 33076359 Free PMC article. Review.
Cited by
-
Divergent synthesis of a thiolate-based α-hydroxytropolone library with a dynamic bioactivity profile.RSC Adv. 2019;9(59):34227-34234. doi: 10.1039/c9ra06383h. Epub 2019 Oct 23. RSC Adv. 2019. PMID: 33042521 Free PMC article.
-
Spectrophotometric determination of α-hydroxytropolone pK a values: A structure-acidity relationship study.Tetrahedron Lett. 2019 Jun 20;60(25):1643-1645. doi: 10.1016/j.tetlet.2019.05.034. Epub 2019 May 20. Tetrahedron Lett. 2019. PMID: 32855576 Free PMC article.
-
Synthesis of aryl-substituted 2-methoxyphenol derivatives from maltol-derived oxidopyrylium cycloadducts through an acid-mediated ring contraction cascade.Chem Commun (Camb). 2020 Mar 14;56(21):3203-3205. doi: 10.1039/c9cc09213g. Epub 2020 Feb 18. Chem Commun (Camb). 2020. PMID: 32068199 Free PMC article.
-
The Exonuclease Activity of Herpes Simplex Virus 1 UL12 Is Required for Production of Viral DNA That Can Be Packaged To Produce Infectious Virus.J Virol. 2017 Nov 14;91(23):e01380-17. doi: 10.1128/JVI.01380-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28956767 Free PMC article.
-
Sensitivity of the C-Terminal Nuclease Domain of Kaposi's Sarcoma-Associated Herpesvirus ORF29 to Two Classes of Active-Site Ligands.Antimicrob Agents Chemother. 2018 Sep 24;62(10):e00233-18. doi: 10.1128/AAC.00233-18. Print 2018 Oct. Antimicrob Agents Chemother. 2018. PMID: 30061278 Free PMC article.
References
-
-
For lead examples relevant to HIV, see: Budihas SR, Gorshkova I, Gaidamakov S, Wamiru A, Bona MK, Parniak MA, Crouch RJ, McMahon JB, Beutler JA, Le Grice SFJ. Nucleic Acids Res. 2005;33:1249. Semenova EA, Johnson AA, Marchand C, Davis DA, Yarchoan R, Pommier Y. Mol. Pharmacol. 2006;69:1454. Himmel DM, Maegley KA, Pauly TA, Bauman JD, Das K, Dharia C, Clark AD, Jr, Ryan K, Hickey MJ, Love RA, Hughes SH, Bergqvist S, Arnold E. Structure. 2009;17:1625.
-
-
-
For lead examples relevant to malaria, see: Omura S, Kasuhiko O, Iwatsuki M, Shiomi K, Masuma R. Jpn. Kokai Tokkyo Koho. 2011 2011084534 A 20110428. Iwatsuki M, Takada S, Mori M, Ishiyama A, Namatame M, Otoguro K, Nishihara-Tsukashima A, Nonaka K, Masuma R, Otoguro O, Shiomi K, Omura S. J. Antibiot. 2011;64:183.
-
-
- Piettre SR, Ganzhorn A, Hoflack J, Islam K, Hornsperger JM. J. Am. Chem. Soc. 1997;119:3201.
-
- Nakagawa Y, Tayama K. Chem.-Biol. Interact. 1998;116:45. - PubMed
-
- Banwell MG, Collis MP, Crisp GT, Lambert JT, Reum ME, Scoble JA. J. Chem. Soc., Chem. Commun. 1989;10:616.
- Zinser H, Sonja H, Foehlisch B. Eur. J. Org. Chem. 2004;6:1344.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

