Transient lack of glucose but not O2 is involved in ischemic postconditioning-induced neuroprotection
- PMID: 23167958
- PMCID: PMC6493356
- DOI: 10.1111/cns.12033
Transient lack of glucose but not O2 is involved in ischemic postconditioning-induced neuroprotection
Abstract
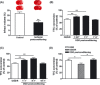
Aim: Cerebral ischemic postconditioning has emerged recently as a kind of endogenous strategy for neuroprotection. We set out to test whether hypoxia or glucose deprivation (GD) would substitute for ischemia in postconditioning.
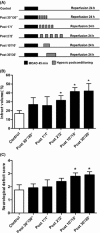
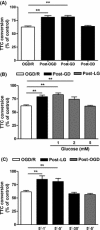
Methods: Adult male C57BL/6J mice were treated with postconditioning evoked by ischemia (bilateral common carotid arteries occlusion) or hypoxia (8% O(2) ) after 45-min middle cerebral arterial occlusion. Corticostriatal slices from mice were subjected to 1-min oxygen-glucose deprivation (OGD), GD, or oxygen deprivation (OD) postconditioning at 5 min after 15-min OGD.
Results: Hypoxic postconditioning did not decrease infarct volume or improve neurologic function at 24 h after reperfusion, while ischemic postconditioning did. Similarly, OGD and GD but not OD postconditioning attenuated the OGD/reperfusion-induced injury in corticostriatal slices. The effective duration of low-glucose (1 mmol/L) postconditioning was longer than that of OGD postconditioning. Moreover, OGD and GD but not OD postconditioning reversed the changes of glutamate, GABA, glutamate transporter-1 protein expression, and glutamine synthetase activity induced by OGD/reperfusion.
Conclusions: These results suggest that the transient lack of glucose but not oxygen plays a key role in ischemic postconditioning-induced neuroprotection, at least partly by regulating glutamate metabolism. Low-glucose postconditioning might be a clinically safe and feasible therapeutic approach against cerebral ischemia/reperfusion injury.
© 2012 Blackwell Publishing Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Postconditioning with isoflurane reduced ischemia-induced brain injury in rats.Anesthesiology. 2008 Jun;108(6):1055-62. doi: 10.1097/ALN.0b013e3181730257. Anesthesiology. 2008. PMID: 18497606 Free PMC article.
-
MCP-induced protein 1 mediates the minocycline-induced neuroprotection against cerebral ischemia/reperfusion injury in vitro and in vivo.J Neuroinflammation. 2015 Feb 27;12:39. doi: 10.1186/s12974-015-0264-1. J Neuroinflammation. 2015. PMID: 25888869 Free PMC article.
-
Experimental Models to Study the Neuroprotection of Acidic Postconditioning Against Cerebral Ischemia.J Vis Exp. 2017 Jul 31;(125):55931. doi: 10.3791/55931. J Vis Exp. 2017. PMID: 28784980 Free PMC article.
-
Neuroprotective Efficacy of an Aminopropyl Carbazole Derivative P7C3-A20 in Ischemic Stroke.CNS Neurosci Ther. 2016 Sep;22(9):782-8. doi: 10.1111/cns.12576. Epub 2016 Jun 23. CNS Neurosci Ther. 2016. PMID: 27333812 Free PMC article.
-
Neuroprotection Promoted by Guanosine Depends on Glutamine Synthetase and Glutamate Transporters Activity in Hippocampal Slices Subjected to Oxygen/Glucose Deprivation.Neurotox Res. 2016 May;29(4):460-8. doi: 10.1007/s12640-015-9595-z. Epub 2016 Feb 8. Neurotox Res. 2016. PMID: 26858177
Cited by
-
Inhibition of G protein-coupled receptor 81 (GPR81) protects against ischemic brain injury.CNS Neurosci Ther. 2015 Mar;21(3):271-9. doi: 10.1111/cns.12362. Epub 2014 Dec 11. CNS Neurosci Ther. 2015. PMID: 25495836 Free PMC article.
-
Neuroprotective Role of Acidosis in Ischemia: Review of the Preclinical Evidence.Mol Neurobiol. 2021 Dec;58(12):6684-6696. doi: 10.1007/s12035-021-02578-5. Epub 2021 Oct 4. Mol Neurobiol. 2021. PMID: 34606050 Review.
-
Platelet-derived microparticles are implicated in remote ischemia conditioning in a rat model of cerebral infarction.CNS Neurosci Ther. 2013 Dec;19(12):917-25. doi: 10.1111/cns.12199. Epub 2013 Nov 4. CNS Neurosci Ther. 2013. PMID: 24267641 Free PMC article.
-
Extracellular visfatin has nicotinamide phosphoribosyltransferase enzymatic activity and is neuroprotective against ischemic injury.CNS Neurosci Ther. 2014 Jun;20(6):539-47. doi: 10.1111/cns.12273. Epub 2014 Apr 21. CNS Neurosci Ther. 2014. PMID: 24750959 Free PMC article.
-
Bakkenolide-IIIa Protects Against Cerebral Damage Via Inhibiting NF-κB Activation.CNS Neurosci Ther. 2015 Dec;21(12):943-52. doi: 10.1111/cns.12470. Epub 2015 Oct 28. CNS Neurosci Ther. 2015. PMID: 26511680 Free PMC article.
References
-
- Pignataro G, Scorziello A, Di Renzo G, Annunziato L. Post‐ischemic brain damage: Effect of ischemic preconditioning and postconditioning and identification of potential candidates for stroke therapy. FEBS J 2009;276:46–57. - PubMed
-
- Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: Ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab 2006;26:1114–1121. - PubMed
-
- Xing B, Chen H, Zhang M, et al. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke 2008;39:2362–2369. - PubMed
-
- Pignataro G, Meller R, Inoue K, et al. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: Ischemic postconditioning. J Cereb Blood Flow Metab 2008;28:232–241. - PubMed
-
- Gao XW, Ren CC, Zhao H. Protective effects of ischemic postconditioning compared with gradual reperfusion or preconditioning. J Neurosci Res 2008;86:2505–2511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

