From cytogenomic to epigenomic profiles: monitoring the biologic behavior of in vitro cultured human bone marrow mesenchymal stem cells
- PMID: 23168092
- PMCID: PMC3580477
- DOI: 10.1186/scrt138
From cytogenomic to epigenomic profiles: monitoring the biologic behavior of in vitro cultured human bone marrow mesenchymal stem cells
Abstract
Introduction: Bone marrow mesenchymal stem cells (BM-MSCs) are multipotent cells that can differentiate into different cell lineages and have emerged as a promising tool for cell-targeted therapies and tissue engineering. Their use in a therapeutic context requires large-scale in vitro expansion, increasing the probability of genetic and epigenetic instabilities. Some evidence shows that an organized program of replicative senescence is triggered in human BM-MSCs (hBM-MSCs) on prolonged in vitro expansion that includes alterations in phenotype, differentiation potential, telomere length, proliferation rates, global gene-expression patterns, and DNA methylation profiles.
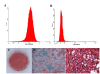
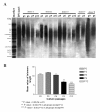
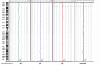
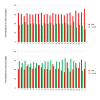
Methods: In this study, we monitored the chromosomal status, the biologic behavior, and the senescence state of hBM-MSCs derived from eight healthy donors at different passages during in vitro propagation. For a more complete picture, the telomere length was also monitored in five of eight donors, whereas the genomic profile was evaluated in three of eight donors by array-comparative genomic hybridization (array-CGH). Finally, an epigenomic profile was delineated and compared between early and late passages, by pooling DNA of hBM-MSCs from four donors.
Results: Our data indicate that long-term culture severely affects the characteristics of hBM-MSCs. All the observed changes (that is, enlarged morphology, decreased number of cell divisions, random loss of genomic regions, telomere shortening) might be regulated by epigenetic modifications. Gene Ontology analysis revealed that specific biologic processes of hBM-MSCs are affected by variations in DNA methylation from early to late passages.
Conclusions: Because we revealed a significant decrease in DNA methylation levels in hBM-MSCs during long-term culture, it is very important to unravel how these modifications can influence the biologic features of hBM-MSCs to keep track of this organized program and also to clarify the conflicting observations on hBM-MSC malignant transformation in the literature.
Figures


Comment in
-
Implications of long-term culture for mesenchymal stem cells: genetic defects or epigenetic regulation?Stem Cell Res Ther. 2012 Dec 20;3(6):54. doi: 10.1186/scrt145. Stem Cell Res Ther. 2012. PMID: 23257053 Free PMC article.
Similar articles
-
Identity, proliferation capacity, genomic stability and novel senescence markers of mesenchymal stem cells isolated from low volume of human bone marrow.Oncotarget. 2016 Mar 8;7(10):10788-802. doi: 10.18632/oncotarget.7456. Oncotarget. 2016. PMID: 26910916 Free PMC article.
-
Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms.Cancer Res. 2007 Oct 1;67(19):9142-9. doi: 10.1158/0008-5472.CAN-06-4690. Cancer Res. 2007. PMID: 17909019
-
In vitro biosafety profile evaluation of multipotent mesenchymal stem cells derived from the bone marrow of sarcoma patients.J Transl Med. 2014 Apr 9;12:95. doi: 10.1186/1479-5876-12-95. J Transl Med. 2014. PMID: 24716831 Free PMC article.
-
The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: a systematic review.J Transl Med. 2019 Nov 29;17(1):397. doi: 10.1186/s12967-019-02136-7. J Transl Med. 2019. PMID: 31783866 Free PMC article.
-
Human Bone Marrow-Derived Mesenchymal Stem Cell Applications in Neurodegenerative Disease Treatment and Integrated Omics Analysis for Successful Stem Cell Therapy.Bioengineering (Basel). 2023 May 22;10(5):621. doi: 10.3390/bioengineering10050621. Bioengineering (Basel). 2023. PMID: 37237691 Free PMC article. Review.
Cited by
-
Mesenchymal stromal cell therapy for damaged retinal ganglion cells, is gold all that glitters?Neural Regen Res. 2019 Nov;14(11):1851-1857. doi: 10.4103/1673-5374.259601. Neural Regen Res. 2019. PMID: 31290434 Free PMC article. Review.
-
Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation.Cell Death Dis. 2013 Dec 5;4(12):e950. doi: 10.1038/cddis.2013.480. Cell Death Dis. 2013. PMID: 24309937 Free PMC article.
-
A model study for the manufacture and validation of clinical-grade deciduous dental pulp stem cells for chronic liver fibrosis treatment.Stem Cell Res Ther. 2020 Mar 25;11(1):134. doi: 10.1186/s13287-020-01630-w. Stem Cell Res Ther. 2020. PMID: 32213198 Free PMC article.
-
Genomic Instabilities, Cellular Senescence, and Aging: In Vitro, In Vivo and Aging-Like Human Syndromes.Front Med (Lausanne). 2018 Apr 17;5:104. doi: 10.3389/fmed.2018.00104. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29719834 Free PMC article. Review.
-
Genetic evaluation of mesenchymal stem cells.Rev Bras Hematol Hemoter. 2014 Jul-Aug;36(4):238-40. doi: 10.1016/j.bjhh.2014.05.014. Epub 2014 Jun 2. Rev Bras Hematol Hemoter. 2014. PMID: 25031159 Free PMC article. No abstract available.
References
-
- Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

