Distinct roles of Ser-764 and Lys-773 at the N terminus of von Willebrand factor in complex assembly with coagulation factor VIII
- PMID: 23168412
- PMCID: PMC3537036
- DOI: 10.1074/jbc.M112.400572
Distinct roles of Ser-764 and Lys-773 at the N terminus of von Willebrand factor in complex assembly with coagulation factor VIII
Abstract
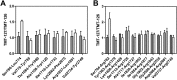
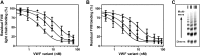
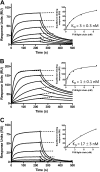
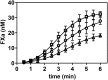
Complex formation between coagulation factor VIII (FVIII) and von Willebrand factor (VWF) is of critical importance to protect FVIII from rapid in vivo clearance and degradation. We have now employed a chemical footprinting approach to identify regions on VWF involved in FVIII binding. To this end, lysine amino acid residues of VWF were chemically modified in the presence of FVIII or activated FVIII, which does not bind VWF. Nano-LC-MS analysis showed that the lysine residues of almost all identified VWF peptides were not differentially modified upon incubation of VWF with FVIII or activated FVIII. However, Lys-773 of peptide Ser-766-Leu-774 was protected from chemical modification in the presence of FVIII. In addition, peptide Ser-764-Arg-782, which comprises the first 19 amino acid residues of mature VWF, showed a differential modification of both Lys-773 and the α-amino group of Ser-764. To verify the role of Lys-773 and the N-terminal Ser-764 in FVIII binding, we employed VWF variants in which either Lys-773 or Ser-764 was replaced with Ala. Surface plasmon resonance analysis and competition studies revealed that VWF(K773A) exhibited reduced binding to FVIII and the FVIII light chain, which harbors the VWF-binding site. In contrast, VWF(S764A) revealed more effective binding to FVIII and the FVIII light chain compared with WT VWF. The results of our study show that the N terminus of VWF is critical for the interaction with FVIII and that Ser-764 and Lys-773 have opposite roles in the binding mechanism.
Figures


References
-
- Schneppenheim R., Budde U. (2011) von Willebrand factor: the complex molecular genetics of a multidomain and multifunctional protein. J. Thromb. Haemost. 9, 209–215 - PubMed
-
- Fay P. J. (2006) Factor VIII structure and function. Int. J. Hematol. 83, 103–108 - PubMed
-
- Morfini M., Mannucci P. M., Tenconi P. M., Longo G., Mazzucconi M. G., Rodeghiero F., Ciavarella N., De Rosa V., Arter A. (1993) Pharmacokinetics of monoclonally-purified and recombinant factor VIII in patients with severe von Willebrand disease. Thromb. Haemost. 70, 270–272 - PubMed
-
- Lenting P. J., Donath M. J., van Mourik J. A., Mertens K. (1994) Identification of a binding site for blood coagulation factor IXa on the light chain of human factor VIII. J. Biol. Chem. 269, 7150–7155 - PubMed
-
- Jacquemin M. (2009) Factor VIII-von Willebrand factor binding defects in autosomal recessive von Willebrand disease type Normandy and in mild hemophilia A. New insights into factor VIII-von Willebrand factor interactions. Acta Haematol. 121, 102–105 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

