Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study
- PMID: 23169319
- PMCID: PMC3572583
- DOI: 10.1002/art.37711
Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study
Abstract
Objective: There is a need for comparative studies to provide evidence-based treatment guidance for biologic agents in rheumatoid arthritis (RA). Therefore, this study was undertaken as the first head-to-head comparison of subcutaneous (SC) abatacept and SC adalimumab, both administered along with background methotrexate (MTX), for the treatment of RA.
Methods: Patients with active RA who were naive to treatment with biologic agents and had an inadequate response to MTX were randomly assigned to receive 125 mg SC abatacept weekly or 40 mg SC adalimumab biweekly, both given in combination with MTX, in a 2-year study. The primary end point was treatment noninferiority, assessed according to the American College of Rheumatology 20% improvement response (ACR20) at 1 year.
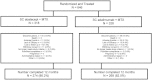
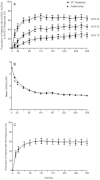
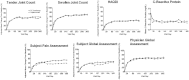
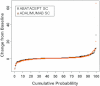
Results: Of the 646 patients who were randomized and treated, 86.2% receiving SC abatacept and 82% receiving SC adalimumab completed 12 months of treatment. At 1 year, 64.8% of patients in the SC abatacept group and 63.4% in the SC adalimumab group demonstrated an ACR20 response; the estimated difference between groups was 1.8% (95% confidence interval -5.6%, 9.2%), thus demonstrating the noninferiority of abatacept compared to adalimumab. All efficacy measures showed similar results and kinetics of response between treatments. The rate of radiographic nonprogression (defined as a total modified Sharp/van der Heijde score [SHS] less than or equal to the smallest detectable change) was 84.8% for SC abatacept-treated patients and 88.6% for SC adalimumab-treated patients, while the mean change from baseline in the total SHS was 0.58 and 0.38, respectively. In the SC abatacept and SC adalimumab groups, the incidence of serious adverse events (SAEs) was 10.1% and 9.1%, respectively, and the rate of serious infections was 2.2% and 2.7%, respectively. In patients treated with SC abatacept, the frequency of discontinuations due to AEs was 3.5% and discontinuations due to SAEs was 1.3%, while in patients treated with SC adalimumab, the frequencies were 6.1% and 3%, respectively. Injection site reactions occurred in 3.8% of patients receiving SC abatacept compared to 9.1% of patients receiving SC adalimumab (P=0.006).
Conclusion: The results demonstrate that SC abatacept and SC adalimumab have comparable efficacy in patients with RA, as shown by similar kinetics of response and comparable inhibition of radiographic progression over 1 year of treatment. The safety was generally similar, other than the occurrence of significantly more local injection site reactions in patients treated with SC adalimumab.
Trial registration: ClinicalTrials.gov NCT00929864.
Copyright © 2013 by the American College of Rheumatology.
Figures
Comment in
-
First face-off in RA biologic therapy declared a draw.Nat Rev Rheumatol. 2013 Jan;9(1):1. doi: 10.1038/nrrheum.2012.222. Epub 2012 Dec 11. Nat Rev Rheumatol. 2013. PMID: 23229451 No abstract available.
References
-
- McInnes IB, O'Dell JR. State-of-the-art: rheumatoid arthritis. Ann Rheum Dis. 2010;69:1898–906. [published erratum appears in Ann Rheum Dis 2011;70:399]. - PubMed
-
- Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. - PMC - PubMed
-
- Knevel R, Schoels M, Huizinga TW, Aletaha D, Burmester GR, Combe B, et al. Current evidence for a strategic approach to the management of rheumatoid arthritis with disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2010;69:987–94. - PubMed
-
- Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. PREMIER Investigators, editor. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. - PubMed
-
- Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti–tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–11. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

