Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody
- PMID: 23169436
- PMCID: PMC3548952
- DOI: 10.1158/1078-0432.CCR-12-2625
Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody
Abstract
Purpose: Results from the first-in-human phase I trial of the anti-programmed death-1 (PD-1) antibody BMS-936558 in patients with treatment-refractory solid tumors, including safety, tolerability, pharmacodynamics, and immunologic correlates, have been previously reported. Here, we provide long-term follow-up on three patients from that trial who sustained objective tumor regressions off therapy, and test the hypothesis that reinduction therapy for late tumor recurrence can be effective.
Experimental design: Three patients with colorectal cancer, renal cell cancer, and melanoma achieved objective responses on an intermittent dosing regimen of BMS-936558. Following cessation of therapy, patients were followed for more than 3 years. A patient with melanoma who experienced a prolonged partial regression followed by tumor recurrence received reinduction therapy.
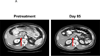
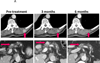
Results: A patient with colorectal cancer experienced a complete response, which is ongoing after 3 years. A patient with renal cell cancer experienced a partial response lasting 3 years off therapy, which converted to a complete response, which is ongoing at 12 months. A patient with melanoma achieved a partial response that was stable for 16 months off therapy; recurrent disease was successfully treated with reinduction anti-PD-1 therapy.
Conclusion: These data represent the most prolonged observation to date of patients with solid tumors responding to anti-PD-1 immunotherapy and the first report of successful reinduction therapy following delayed tumor progression. They underscore the potential for immune checkpoint blockade with anti-PD-1 to reset the equilibrium between tumor and the host immune system.
©2012 AACR.
Conflict of interest statement
EJL: none
WHS: compensated consultant for Genentech and Merck; honoraria from Prometheus
CGD: compensated consultant for Bristol-Myers Squibb, Dendreon, Janssen, and ImmuneXcite; stock or other ownership interest in Amplimmune; honoraria from Bristol-Myers Squibb, Dendreon, Janssen; other remuneration (patent licensing) from Amplimmune and Bristol-Myers Squibb
IW: none
JMT: research funding from Bristol-Myers Squibb; travel reimbursement from Bristol-Myers Squibb
RAA: compensated consultant, contract for service, research funding and other remuneration from Bristol-Myers Squibb
HX: none
SY: employment (compensated, research scientist) and stock holdings or other ownership at Amplimmune
AP: none
LC: none
DMP: uncompensated consultant for Bristol-Myers Squibb, compensated consultant for Amplimmune and ImmuneXcite
JRB: uncompensated consultant for Bristol-Myers Squibb, compensated consultant for Genentech and Eli Lilly
SLT: uncompensated consultant for Bristol-Myers Squibb, compensated consultant for Amplimmune; research funding from Bristol-Myers Squibb; travel reimbursement from Bristol-Myers Squibb
Figures







References
-
- Kamino H, Tam ST. Immunoperoxidase technique modified by counterstain with azure B as a diagnostic aid in evaluating heavily pigmented melanocytic neoplasms. J Cutan Pathol. 1991;18(6):436–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

