Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial
- PMID: 23169517
- PMCID: PMC4110249
- DOI: 10.1200/JCO.2012.45.0494
Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial
Abstract
Purpose: Cabozantinib (XL184) is an orally bioavailable tyrosine kinase inhibitor with activity against MET and vascular endothelial growth factor receptor 2. We evaluated the activity of cabozantinib in patients with castration-resistant prostate cancer (CRPC) in a phase II randomized discontinuation trial with an expansion cohort.
Patients and methods: Patients received 100 mg of cabozantinib daily. Those with stable disease per RECIST at 12 weeks were randomly assigned to cabozantinib or placebo. Primary end points were objective response rate at 12 weeks and progression-free survival (PFS) after random assignment.
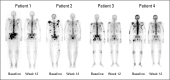
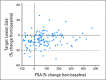
Results: One hundred seventy-one men with CRPC were enrolled. Random assignment was halted early based on the observed activity of cabozantinib. Seventy-two percent of patients had regression in soft tissue lesions, whereas 68% of evaluable patients had improvement on bone scan, including complete resolution in 12%. The objective response rate at 12 weeks was 5%, with stable disease in 75% of patients. Thirty-one patients with stable disease at week 12 were randomly assigned. Median PFS was 23.9 weeks (95% CI, 10.7 to 62.4 weeks) with cabozantinib and 5.9 weeks (95% CI, 5.4 to 6.6 weeks) with placebo (hazard ratio, 0.12; P < .001). Serum total alkaline phosphatase and plasma cross-linked C-terminal telopeptide of type I collagen were reduced by ≥ 50% in 57% of evaluable patients. On retrospective review, bone pain improved in 67% of evaluable patients, with a decrease in narcotic use in 56%. The most common grade 3 adverse events were fatigue (16%), hypertension (12%), and hand-foot syndrome (8%).
Conclusion: Cabozantinib has clinical activity in men with CRPC, including reduction of soft tissue lesions, improvement in PFS, resolution of bone scans, and reductions in bone turnover markers, pain, and narcotic use.
Trial registration: ClinicalTrials.gov NCT00940225.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures





Comment in
-
Cabozantinib in prostate cancer: the beginning of a precision paradigm?J Clin Oncol. 2013 Feb 1;31(4):401-3. doi: 10.1200/JCO.2012.46.6185. Epub 2012 Dec 17. J Clin Oncol. 2013. PMID: 23248244 No abstract available.
-
Commentary on "Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial." Smith DC, Smith MR, Sweeney C, Elfiky AA, Logothetis C, Corn PG, Vogelzang NJ, Small EJ, Harzstark AL, Gordon MS, Vaishampayan UN, Haas NB, Spira AI, Lara PN Jr, Lin CC, Srinivas S, Sella A, SchoffskiSchöffski P, Scheffold C, Weitzman AL, Hussain M, University of Michigan, Ann Arbor, MI. J Clin Oncol 2013;31(4):412-9. doi: 10.1200/JCO.2012.45.0494. Epub 2012 Nov 19.Urol Oncol. 2013 Nov;31(8):1848. doi: 10.1016/j.urolonc.2013.07.012. Urol Oncol. 2013. PMID: 24210087
References
-
- Pisters LL, Troncoso P, Zhau HE, et al. c-met proto-oncogene expression in benign and malignant human prostate tissues. J Urol. 1995;154:293–298. - PubMed
-
- Knudsen BS, Gmyrek GA, Inra J, et al. High expression of the Met receptor in prostate cancer metastasis to bone. Urology. 2002;60:1113–1117. - PubMed
-
- Humphrey PA, Halabi S, Picus J, et al. Prognostic significance of plasma scatter factor/hepatocyte growth factor levels in patients with metastatic hormone-refractory prostate cancer: Results from cancer and leukemia group B 150005/9480. Clin Genitourin Cancer. 2006;4:269–274. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

