Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial
- PMID: 23169523
- PMCID: PMC3530688
- DOI: 10.1200/JCO.2012.44.6518
Parenteral hydration in patients with advanced cancer: a multicenter, double-blind, placebo-controlled randomized trial
Abstract
Purpose: The vast majority of patients with cancer at the end of life receive parenteral hydration in hospitals and no hydration in hospice, with limited evidence supporting either practice. In this randomized controlled trial, we determined the effect of hydration on symptoms associated with dehydration, quality of life, and survival in patients with advanced cancer.
Patients and methods: We randomly assigned 129 patients with cancer from six hospices to receive parenteral hydration (normal saline 1 L per day) or placebo (normal saline 100 mL per day) daily over 4 hours. The primary outcome was change in the sum of four dehydration symptoms (fatigue, myoclonus, sedation and hallucinations, 0 = best and 40 = worst possible) between day 4 and baseline. Secondary outcomes included Edmonton Symptom Assessment Scale (ESAS), Memorial Delirium Assessment Scale (MDAS), Nursing Delirium Screening Scale (NuDESC), Unified Myoclonus Rating Scale (UMRS), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Dehydration Assessment Scale, creatinine, urea, and overall survival. Intention-to-treat analysis was conducted to examine the change by day 4 ± 2 and day 7 ± 2 between groups.
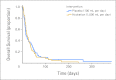
Results: The hydration (n = 63) and placebo (n = 66) groups had similar baseline characteristics. We found no significant differences between the two groups for change in the sum of four dehydration symptoms (-3.3 v -2.8, P = .77), ESAS (all nonsignificant), MDAS (1 v 3.5, P = .084), NuDESC (0 v 0, P = .13), and UMRS (0 v 0, P = .54) by day 4. Results for day 7, including FACIT-F, were similar. Overall survival did not differ between the two groups (median, 21 v 15 days, P = .83).
Conclusion: Hydration at 1 L per day did not improve symptoms, quality of life, or survival compared with placebo.
Trial registration: ClinicalTrials.gov NCT00423722.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
ACP Journal Club. Parenteral hydration did not improve dehydration or quality of life in advanced cancer.Ann Intern Med. 2013 Mar 19;158(6):JC10. doi: 10.7326/0003-4819-158-6-201303190-02010. Ann Intern Med. 2013. PMID: 23552960 No abstract available.
References
-
- Dalal S, Del Fabbro E, Bruera E. Is there a role for hydration at the end of life? Curr Opin Support Palliat Care. 2009;3:72–78. - PubMed
-
- Dalal S, Bruera E. Dehydration in cancer patients: To treat or not to treat. J Support Oncol, 483. 2004;2:467–479. - PubMed
-
- Bruera E, Sala R, Rico MA, et al. Effects of parenteral hydration in terminally ill cancer patients: A preliminary study. J Clin Oncol. 2005;23:2366–2371. - PubMed
-
- de Stoutz ND, Bruera E, Suarez-Almazor M. Opioid rotation for toxicity reduction in terminal cancer patients. J Pain Symptom Manage. 1995;10:378–384. - PubMed
-
- Bruera E, Franco JJ, Maltoni M, et al. Changing pattern of agitated impaired mental status in patients with advanced cancer: Association with cognitive monitoring, hydration, and opioid rotation. J Pain Symptom Manage. 1995;10:287–291. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical