A very low carbohydrate ketogenic diet prevents the progression of hepatic steatosis caused by hyperglycemia in a juvenile obese mouse model
- PMID: 23169538
- PMCID: PMC3506983
- DOI: 10.1038/nutd.2012.24
A very low carbohydrate ketogenic diet prevents the progression of hepatic steatosis caused by hyperglycemia in a juvenile obese mouse model
Abstract
Objective: To investigate whether the improvement in hyperglycemia by dietary control influences hyperglycemia-induced pathologies in tissues of juvenile obese (ob/ob) mice.
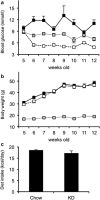
Design: Five-week-old ob/ob mice were fed a very low carbohydrate ketogenic diet (KD) for 7 weeks. The blood glucose levels and body weight were monitored during this period. Biochemical parameters in the serum and tissue pathologies of the mice were analyzed at the end of the 7-week period.
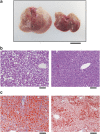
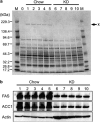
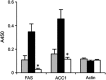
Results: The hyperglycemic phenotype of the ob/ob mice was improved by KD feeding for 7 weeks. Surprisingly, we found that KD feeding also drastically reduced the hepatic steatosis phenotype in ob/ob mice, while their obesity phenotype was unaltered. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis revealed that several proteins found in the liver of ob/ob mice fed a regular chow diet were undetectable after being fed KD. Liquid chromatography with tandem mass spectrometry (LC-MS/MS) MASCOT search and western blot analysis revealed that the proteins absent from the mice fed KD included fatty acid synthase (FAS) and acetyl-CoA carboxylase 1 (ACC1), which are key enzymes for lipogenesis in the liver. Fatty acid analysis supported the results because the ratio of C18:1, which is a major product of lipogenesis, was reduced by KD feeding. However, C18:2, which cannot be synthesized in mammalian cells but is present in the KD, was found to be a major component in the liver of KD-fed ob/ob mice.
Conclusion: Hyperglycemia promotes hepatic steatosis via the lipogenic pathway in the liver of juvenile ob/ob mice. However, the development of steatosis is prevented by feeding KD owing to an improvement in hyperglycemia. We found that the progression of steatosis is reflected by the composition of fatty acids in the total lipids of the liver and serum.
Figures
Similar articles
-
Methionine restriction prevents the progression of hepatic steatosis in leptin-deficient obese mice.Metabolism. 2013 Nov;62(11):1651-61. doi: 10.1016/j.metabol.2013.06.012. Epub 2013 Aug 5. Metabolism. 2013. PMID: 23928105
-
A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss.Am J Physiol Endocrinol Metab. 2009 Nov;297(5):E1197-204. doi: 10.1152/ajpendo.00357.2009. Epub 2009 Sep 8. Am J Physiol Endocrinol Metab. 2009. PMID: 19738035 Free PMC article.
-
Altered expression of O-GlcNAc-modified proteins in a mouse model whose glycemic status is controlled by a low carbohydrate ketogenic diet.Glycoconj J. 2013 Nov;30(8):781-9. doi: 10.1007/s10719-013-9482-x. Epub 2013 Jun 21. Glycoconj J. 2013. PMID: 23793825
-
Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice.Diabetes. 2006 Aug;55(8):2159-70. doi: 10.2337/db06-0200. Diabetes. 2006. PMID: 16873678
-
Current biochemical studies of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis suggest a new therapeutic approach.Am J Gastroenterol. 2003 Sep;98(9):2093-7. doi: 10.1111/j.1572-0241.2003.07670.x. Am J Gastroenterol. 2003. PMID: 14499793 Review.
Cited by
-
A low-carbohydrate ketogenic diet induces the expression of very-low-density lipoprotein receptor in liver and affects its associated metabolic abnormalities.NPJ Sci Food. 2019 Dec 2;3:25. doi: 10.1038/s41538-019-0058-4. eCollection 2019. NPJ Sci Food. 2019. PMID: 31815184 Free PMC article.
-
Activation of G protein-coupled receptors by ketone bodies: Clinical implication of the ketogenic diet in metabolic disorders.Front Endocrinol (Lausanne). 2022 Oct 20;13:972890. doi: 10.3389/fendo.2022.972890. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36339405 Free PMC article. Review.
-
Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus.Nutr Diabetes. 2016 Sep 19;6(9):e230. doi: 10.1038/nutd.2016.36. Nutr Diabetes. 2016. PMID: 27643725 Free PMC article. Clinical Trial.
-
Induction of specific adaptive immune responses by immunization with newly designed artificial glycosphingolipids.Sci Rep. 2019 Dec 11;9(1):18803. doi: 10.1038/s41598-019-55088-9. Sci Rep. 2019. PMID: 31827147 Free PMC article.
-
Hepatic safety profile of pancreatic cancer‑bearing mice fed a ketogenic diet in combination with gemcitabine.Oncol Lett. 2023 Sep 22;26(5):479. doi: 10.3892/ol.2023.14067. eCollection 2023 Nov. Oncol Lett. 2023. PMID: 37818128 Free PMC article.
References
-
- Kilpatrick ES, Rigby AS, Atkin SL. The Diabetes Control and Complications Trial: the gift that keeps giving. Nat Rev Endocrinol. 2009;5:537–545. - PubMed
-
- Stolar M. Glycemic control and complications in type 2 diabetes mellitus. Am J Med. 2010;123:S3–S11. - PubMed
-
- Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes. 2003;52:2867–2873. - PubMed
-
- Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

