Immunohistochemical and functional characterization of peptide, organic cation, neutral and basic amino acid, and monocarboxylate drug transporters in human ocular tissues
- PMID: 23169611
- PMCID: PMC3558866
- DOI: 10.1124/dmd.112.045674
Immunohistochemical and functional characterization of peptide, organic cation, neutral and basic amino acid, and monocarboxylate drug transporters in human ocular tissues
Retraction in
-
Notice of retraction.Drug Metab Dispos. 2015 Feb;43(2):234. doi: 10.1124/dmd.114.045674err. Drug Metab Dispos. 2015. PMID: 25537846 Free PMC article. No abstract available.
Abstract
Since there is paucity of information on solute transporters in human ocular tissues, the aim of this study was immunohistochemical and functional characterization of peptide transporters (PEPT), organic cation transporters (OCTs), neutral and basic amino acid transporters (ATB(0,+)), and monocarboxylate transporters (MCTs) in human ocular barriers. Immunohistochemical localization of transporters was achieved using 5-µm-thick paraffin-embedded sections of whole human eyes. In vitro transport studies were carried out across human cornea and sclera-choroid-retinal pigment epithelium (SCRPE) using a cassette of specific substrates in the presence and absence of inhibitors to determine the role of transporters in transtissue solute delivery. Immunohistochemistry showed the expression of PEPT-1, PEPT-2, ATB(0,+), OCT-1, OCT-2, MCT-1, and MCT-3 in human ocular tissues. PEPT-1, PEPT-2, OCT-1, MCT-1, and ATB(0,+) expression was evident in the cornea, conjunctiva, ciliary epithelium, and neural retina. Expression of PEPT-1, PEPT-2, and OCT-1 was evident in choroid tissue as well. OCT-2 expression could be seen in the corneal and conjunctival epithelia, whereas MCT-3 expression was confined to the RPE layer. OCT-2 expression was evident in conjunctival blood vessel walls, whereas PEPT-1, PEPT-2, and OCT-1 were expressed in the choroid. Preliminary transport studies indicated inward transport of Gly-Sar (PEPT substrate), 1-methyl-4-phenylpyridinium (MPP+) (OCT substrate), and l-tryptophan (ATB(0,+) substrate) across cornea as well as SCRPE. For phenylacetic acid (MCT substrate), transporter-mediated inward transport across the cornea and outward transport across SCRPE were evident. Thus, PEPT, OCT, and ATB(0,+) are influx transporters present in human ocular barriers, and they can potentially be used for transporter-guided retinal drug delivery after topical, transscleral, and systemic administrations.
Figures


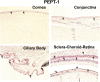




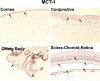



Comment in
-
Findings of Research Misconduct.Fed Regist. 2018 Dec 6;83(234):62875. Fed Regist. 2018. PMID: 30556543 Free PMC article. No abstract available.
References
-
- Anand BS, Mitra AK. (2002) Mechanism of corneal permeation of L-valyl ester of acyclovir: targeting the oligopeptide transporter on the rabbit cornea. Pharm Res 19:1194–1202 - PubMed
-
- Basu SK, Haworth IS, Bolger MB, Lee VH. (1998) Proton-driven dipeptide uptake in primary cultured rabbit conjunctival epithelial cells. Invest Ophthalmol Vis Sci 39:2365–2373 - PubMed
-
- Biegel A, Knütter I, Hartrodt B, et al. (2006) The renal type H+/peptide symporter PEPT2: structure-affinity relationships. Amino Acids 31:137–156 - PubMed
-
- Brandsch M, Knütter I, Thunecke F, Hartrodt B, Born I, Börner V, Hirche F, Fischer G, Neubert K. (1999) Decisive structural determinants for the interaction of proline derivatives with the intestinal H+/peptide symporter. Eur J Biochem 266:502–508 - PubMed
-
- Chidlow G, Wood JP, Graham M, Osborne NN. (2005) Expression of monocarboxylate transporters in rat ocular tissues. Am J Physiol Cell Physiol 288:C416–C428 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

