Ultrasensitivity of the Bacillus subtilis sporulation decision
- PMID: 23169620
- PMCID: PMC3528541
- DOI: 10.1073/pnas.1213974109
Ultrasensitivity of the Bacillus subtilis sporulation decision
Abstract
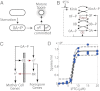
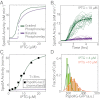
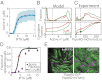
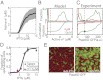
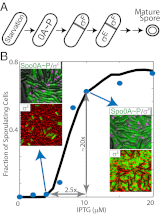
Starving Bacillus subtilis cells execute a gene expression program resulting in the formation of stress-resistant spores. Sporulation master regulator, Spo0A, is activated by a phosphorelay and controls the expression of a multitude of genes, including the forespore-specific sigma factor σ(F) and the mother cell-specific sigma factor σ(E). Identification of the system-level mechanism of the sporulation decision is hindered by a lack of direct control over Spo0A activity. This limitation can be overcome by using a synthetic system in which Spo0A activation is controlled by inducing expression of phosphorelay kinase KinA. This induction results in a switch-like increase in the number of sporulating cells at a threshold of KinA. Using a combination of mathematical modeling and single-cell microscopy, we investigate the origin and physiological significance of this ultrasensitive threshold. The results indicate that the phosphorelay is unable to achieve a sufficiently fast and ultrasensitive response via its positive feedback architecture, suggesting that the sporulation decision is made downstream. In contrast, activation of σ(F) in the forespore and of σ(E) in the mother cell compartments occurs via a cascade of coherent feed-forward loops, and thereby can produce fast and ultrasensitive responses as a result of KinA induction. Unlike σ(F) activation, σ(E) activation in the mother cell compartment only occurs above the KinA threshold, resulting in completion of sporulation. Thus, ultrasensitive σ(E) activation explains the KinA threshold for sporulation induction. We therefore infer that under uncertain conditions, cells initiate sporulation but postpone making the sporulation decision to average stochastic fluctuations and to achieve a robust population response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

