Combination of glycolysis inhibition with chemotherapy results in an antitumor immune response
- PMID: 23169636
- PMCID: PMC3523878
- DOI: 10.1073/pnas.1206360109
Combination of glycolysis inhibition with chemotherapy results in an antitumor immune response
Abstract
Most DNA-damaging agents are weak inducers of an anticancer immune response. Increased glycolysis is one of the best-described hallmarks of tumor cells; therefore, we investigated the impact of glycolysis inhibition, using 2-deoxyglucose (2DG), in combination with cytotoxic agents on the induction of immunogenic cell death. We demonstrated that 2DG synergized with etoposide-induced cytotoxicity and significantly increased the life span of immunocompetent mice but not immunodeficient mice. We then established that only cotreated cells induced an efficient tumor-specific T-cell activation ex vivo and that tumor antigen-specific T cells could only be isolated from cotreated animals. In addition, only when mice were immunized with cotreated dead tumor cells could they be protected (vaccinated) from a subsequent challenge using the same tumor in viable form. Finally, we demonstrated that this effect was at least partially mediated through ERp57/calreticulin exposure on the plasma membrane. These data identify that the targeting of glycolysis can convert conventional tolerogenic cancer cell death stimuli into immunogenic ones, thus creating new strategies for immunogenic chemotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

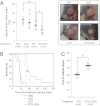

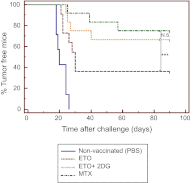

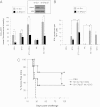
References
-
- Vander Heiden MG. Targeting cancer metabolism: A therapeutic window opens. Nat Rev Drug Discov. 2011;10(9):671–684. - PubMed
-
- El Mjiyad N, Caro-Maldonado A, Ramírez-Peinado S, Muñoz-Pinedo C. Sugar-free approaches to cancer cell killing. Oncogene. 2011;30(3):253–264. - PubMed
-
- Kurtoglu M, et al. Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol Cancer Ther. 2007;6(11):3049–3058. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

