SUMOylation silences heterodimeric TASK potassium channels containing K2P1 subunits in cerebellar granule neurons
- PMID: 23169818
- PMCID: PMC3876883
- DOI: 10.1126/scisignal.2003431
SUMOylation silences heterodimeric TASK potassium channels containing K2P1 subunits in cerebellar granule neurons
Abstract
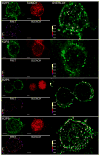
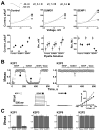
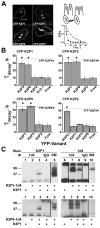
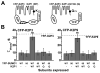
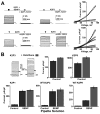
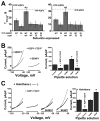
The standing outward K(+) current (IKso) governs the response of cerebellar granule neurons to natural and medicinal stimuli including volatile anesthetics. We showed that SUMOylation silenced half of IKso at the surface of cerebellar granule neurons because the underlying channels were heterodimeric assemblies of K2P1, a subunit subject to SUMOylation, and the TASK (two-P domain, acid-sensitive K(+)) channel subunits K2P3 or K2P9. The heterodimeric channels comprised the acid-sensitive portion of IKso and mediated its response to halothane. We anticipate that SUMOylation also influences sensation and homeostatic mechanisms in mammals through TASK channels formed with K2P1.
Figures

Similar articles
-
Differential expression of two-pore domain potassium channels in rat cerebellar granule neurons.Biochem Biophys Res Commun. 2014 Oct 31;453(4):754-60. doi: 10.1016/j.bbrc.2014.10.012. Epub 2014 Oct 12. Biochem Biophys Res Commun. 2014. PMID: 25305496
-
K+-dependent cerebellar granule neuron apoptosis. Role of task leak K+ channels.J Biol Chem. 2003 Aug 22;278(34):32068-76. doi: 10.1074/jbc.M302631200. Epub 2003 Jun 3. J Biol Chem. 2003. PMID: 12783883
-
Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons.J Neurosci. 2003 Jul 23;23(16):6460-9. doi: 10.1523/JNEUROSCI.23-16-06460.2003. J Neurosci. 2003. PMID: 12878686 Free PMC article.
-
Wrestling with SUMO in a new arena.Sci STKE. 2005 Jun 28;2005(290):pe32. doi: 10.1126/stke.2902005pe32. Sci STKE. 2005. PMID: 15985640 Review.
-
The 2P-domain K+ channels: role in apoptosis and tumorigenesis.Pflugers Arch. 2004 Jun;448(3):261-73. doi: 10.1007/s00424-004-1255-8. Epub 2004 May 5. Pflugers Arch. 2004. PMID: 15133669 Review.
Cited by
-
TWIK-1 BAC-GFP Transgenic Mice, an Animal Model for TWIK-1 Expression.Cells. 2021 Oct 14;10(10):2751. doi: 10.3390/cells10102751. Cells. 2021. PMID: 34685731 Free PMC article.
-
CG4928 Is Vital for Renal Function in Fruit Flies and Membrane Potential in Cells: A First In-Depth Characterization of the Putative Solute Carrier UNC93A.Front Cell Dev Biol. 2020 Oct 14;8:580291. doi: 10.3389/fcell.2020.580291. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33163493 Free PMC article.
-
SUMOylation and calcium signalling: potential roles in the brain and beyond.Neuronal Signal. 2017 Jul 19;1(3):NS20160010. doi: 10.1042/NS20160010. eCollection 2017 Aug. Neuronal Signal. 2017. PMID: 32714579 Free PMC article. Review.
-
SUMOylating Two Distinct Sites on the A-type Potassium Channel, Kv4.2, Increases Surface Expression and Decreases Current Amplitude.Front Mol Neurosci. 2019 May 31;12:144. doi: 10.3389/fnmol.2019.00144. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31213982 Free PMC article.
-
Alterations in SUMOylation of the hyperpolarization-activated cyclic nucleotide-gated ion channel 2 during persistent inflammation.Eur J Pain. 2020 Sep;24(8):1517-1536. doi: 10.1002/ejp.1606. Epub 2020 Jun 14. Eur J Pain. 2020. PMID: 32446289 Free PMC article.
References
-
- Goldstein SAN, Bockenhauer D, O’Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175. - PubMed
-
- Plant LD, Bayliss DA, Kim D, Lesage F, Goldstein SAN. IUPHAR database. 2009.
-
- Ketchum KA, Joiner WJ, Sellers AJ, Kaczmarek LK, Goldstein SAN. A new family of outwardly-rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995;376:690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

