Interaction of lipocalin 2, transferrin, and siderophores determines the replicative niche of Klebsiella pneumoniae during pneumonia
- PMID: 23169997
- PMCID: PMC3509427
- DOI: 10.1128/mBio.00224-11
Interaction of lipocalin 2, transferrin, and siderophores determines the replicative niche of Klebsiella pneumoniae during pneumonia
Abstract
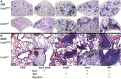
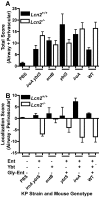
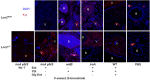
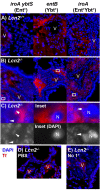
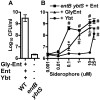
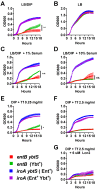
Pathogenic bacteria require iron for replication within their host. Klebsiella pneumoniae and other Gram-negative pathogens produce the prototypical siderophore enterobactin (Ent) to scavenge iron in vivo. In response, mucosal surfaces secrete lipocalin 2 (Lcn2), an innate immune protein that binds Ent to disrupt bacterial iron acquisition and promote acute inflammation during colonization. A subset of K. pneumoniae isolates attempt to evade Lcn2 by producing glycosylated Ent (Gly-Ent, salmochelin) or the alternative siderophore yersiniabactin (Ybt). However, these siderophores are not functionally equivalent and differ in their abilities to promote growth in the upper respiratory tract, lungs, and serum. To understand how Lcn2 exploits functional differences between siderophores, isogenic mutants of an Ent(+) Gly-Ent(+) Ybt(+) K. pneumoniae strain were inoculated into Lcn2(+/+) and Lcn2(-/-) mice, and the pattern of pneumonia was examined. Lcn2 effectively protected against the iroA ybtS mutant (Ent(+) Gly-Ent(-) Ybt(-)). Lcn2(+/+) mice had small foci of pneumonia, whereas Lcn2(-/-) mice had many bacteria in the perivascular space. The entB mutant (Ent(-) Ybt(+) Gly-Ent(-)) caused moderate bronchopneumonia but did not invade the transferrin-containing perivascular space. Accordingly, transferrin blocked Ybt-dependent growth in vitro. The wild type and the iroA mutant, which both produce Ent and Ybt, had a mixed phenotype, causing a moderate bronchopneumonia in Lcn2(+/+) mice and perivascular overgrowth in Lcn2(-/-) mice. Together, these data indicate that Lcn2, in combination with transferrin, confines K. pneumoniae to the airways and prevents invasion into tissue containing the pulmonary vasculature.
Importance: Gram-negative bacteria are a common cause of severe hospital-acquired infections. To cause disease, they must obtain iron and secrete the small molecule enterobactin to do so. Animal models of pneumonia using Klebsiella pneumoniae indicate that enterobactin promotes severe disease. Accordingly, the host defense protein lipocalin 2 exploits this common target by binding enterobactin and disrupting its function. However, pathogenic bacteria often make additional siderophores that lipocalin 2 cannot bind, such as yersiniabactin, which could make this host defense ineffective. This work compares the pattern and severity of pneumonia caused by K. pneumoniae based on which siderophores it produces. The results indicate that enterobactin promotes growth around blood vessels that are rich in the iron-binding protein transferrin, but yersiniabactin does not. Together, transferrin and lipocalin 2 protect this space against all types of K. pneumoniae tested. Therefore, the ability to acquire iron determines where bacteria can grow in the lung.
Figures
References
-
- Earhart CF. 1996. Uptake and metabolism of iron and molybdenum. In Neidhart F, E. coli and salmonella. ASM Press, Washington, DC
-
- Nairz M, Schroll A, Sonnweber T, Weiss G. 2010. The struggle for iron—a metal at the host-pathogen interface. Cell. Microbiol. 12:1691–1702 - PubMed
-
- Skaar EP. 2010. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 6:e1000949 http://dx.doi.org/doi:10.1371/journal.ppat.1000949 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
