Philopatry drives genetic differentiation in an island archipelago: comparative population genetics of Galapagos Nazca boobies (Sula granti) and great frigatebirds (Fregata minor)
- PMID: 23170212
- PMCID: PMC3501629
- DOI: 10.1002/ece3.386
Philopatry drives genetic differentiation in an island archipelago: comparative population genetics of Galapagos Nazca boobies (Sula granti) and great frigatebirds (Fregata minor)
Abstract
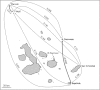
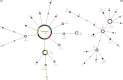
Seabirds are considered highly mobile, able to fly great distances with few apparent barriers to dispersal. However, it is often the case that seabird populations exhibit strong population genetic structure despite their potential vagility. Here we show that Galapagos Nazca booby (Sula granti) populations are substantially differentiated, even within the small geographic scale of this archipelago. On the other hand, Galapagos great frigatebird (Fregata minor) populations do not show any genetic structure. We characterized the genetic differentiation by sampling five colonies of both species in the Galapagos archipelago and analyzing eight microsatellite loci and three mitochondrial genes. Using an F-statistic approach on the multilocus data, we found significant differentiation between nearly all island pairs of Nazca booby populations and a Bayesian clustering analysis provided support for three distinct genetic clusters. Mitochondrial DNA showed less differentiation of Nazca booby colonies; only Nazca boobies from the island of Darwin were significantly differentiated from individuals throughout the rest of the archipelago. Great frigatebird populations showed little to no evidence for genetic differentiation at the same scale. Only two island pairs (Darwin - Wolf, N. Seymour - Wolf) were significantly differentiated using the multilocus data, and only two island pairs had statistically significant φ(ST) values (N. Seymour - Darwin, N. Seymour - Wolf) according to the mitochondrial data. There was no significant pattern of isolation by distance for either species calculated using both markers. Seven of the ten Nazca booby migration rates calculated between island pairs were in the south or southeast to north or northwest direction. The population differentiation found among Galapagos Nazca booby colonies, but not great frigatebird colonies, is most likely due to differences in natal and breeding philopatry.
Keywords: Galapagos; natal philopatry; population genetics; seabird.
Figures

References
-
- Anderson DJ. Differential responses of boobies and other seabirds in the Galapagos to the 1986–1987 El Niño Southern Oscillation event. Mar. Ecol. Prog. Ser. 1989;52:209–216.
-
- Arbogast BS, Drovetski SV, Curry RL, Boag PT, Seutin G, Grant PR, et al. The origin and diversification of Galapaogs Mockingbirds. Evolution. 2006;62:370–382. - PubMed
-
- Bohonak AJ. IBD (Isolation By Distance): a program for analyses of isolation by distance. J. Hered. 2002;93:153–154. - PubMed
-
- Bollmer JL, Whiteman NK, Cannon MD, Bednarz J, Parker T, de Vries PG. Population genetics of the Galapagos Hawk (Buteo galapagoesnsis): genetic monomorphism within isolated populations. Auk. 2005;122:1210–1224.
-
- Bollmer JL, Kimball RT, Whiteman NK, Sarasola JH, Parker PG. Phylogeography of the Galápagos hawk (Buteo galapagoensis): a recent arrival to the Galápagos Islands. Mol. Phylogenet. Evol. 2006;39:237–247. - PubMed
LinkOut - more resources
Full Text Sources

