Multiplicity of Salmonella entry mechanisms, a new paradigm for Salmonella pathogenesis
- PMID: 23170225
- PMCID: PMC3496970
- DOI: 10.1002/mbo3.28
Multiplicity of Salmonella entry mechanisms, a new paradigm for Salmonella pathogenesis
Abstract
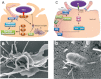
The Salmonella enterica species includes about 2600 diverse serotypes, most of which cause a wide range of food- and water-borne diseases ranging from self-limiting gastroenteritis to typhoid fever in both humans and animals. Moreover, some serotypes are restricted to a few animal species, whereas other serotypes are able to infect plants as well as cold- and warm-blooded animals. An essential feature of the pathogenicity of Salmonella is its capacity to cross a number of barriers requiring invasion of a large variety of phagocytic and nonphagocytic cells. The aim of this review is to describe the different entry pathways used by Salmonella serotypes to enter different nonphagocytic cell types. Until recently, it was accepted that Salmonella invasion of eukaryotic cells required only the type III secretion system (T3SS) encoded by the Salmonella pathogenicity island-1. However, recent evidence shows that Salmonella can cause infection in a T3SS-1-independent manner. Currently, two outer membrane proteins Rck and PagN have been clearly identified as Salmonella invasins. As Rck mediates a Zipper-like entry mechanism, Salmonella is therefore the first bacterium shown to be able to induce both Zipper and Trigger mechanisms to invade host cells. In addition to these known entry pathways, recent data have shown that unknown entry routes could be used according to the serotype, the host and the cell type considered, inducing either Zipper-like or Trigger-like entry processes. The new paradigm presented here should change our classic view of Salmonella pathogenicity. It could also modify our understanding of the mechanisms leading to the different Salmonella-induced diseases and to Salmonella-host specificity.
Keywords: Adhesion; Salmonella; Trigger; Zipper; invasin; invasion; type III secretion system.
Figures
References
-
- Bakowski MA, Cirulis JT, Brown NF, Finlay BB, Brumell JH. SopD acts cooperatively with SopB during Salmonella enterica serovar Typhimurium invasion. Cell Microbiol. 2007;9:2839–2855. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

