Flavonone treatment reverses airway inflammation and remodelling in an asthma murine model
- PMID: 23170811
- PMCID: PMC3605879
- DOI: 10.1111/bph.12062
Flavonone treatment reverses airway inflammation and remodelling in an asthma murine model
Abstract
Background and purpose: Asthma is an inflammatory disease that involves airway hyperresponsiveness and remodelling. Flavonoids have been associated to anti-inflammatory and antioxidant activities and may represent a potential therapeutic treatment of asthma. Our aim was to evaluate the effects of the sakuranetin treatment in several aspects of experimental asthma model in mice.
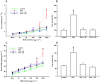
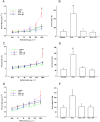
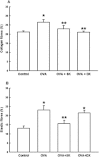
Experimental approach: Male BALB/c mice received ovalbumin (i.p.) on days 0 and 14, and were challenged with aerolized ovalbumin 1% on days 24, 26 and 28. Ovalbumin-sensitized animals received vehicle (saline and dimethyl sulfoxide, DMSO), sakuranetin (20 mg kg(-1) per mice) or dexamethasone (5 mg kg(-1) per mice) daily beginning from 24th to 29th day. Control group received saline inhalation and nasal drop vehicle. On day 29, we determined the airway hyperresponsiveness, inflammation and remodelling as well as specific IgE antibody. RANTES, IL-5, IL-4, Eotaxin, IL-10, TNF-α, IFN-γ and GMC-SF content in lung homogenate was performed by Bioplex assay, and 8-isoprostane and NF-kB activations were visualized in inflammatory cells by immunohistochemistry.
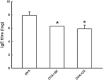
Key results: We have demonstrated that sakuranetin treatment attenuated airway hyperresponsiveness, inflammation and remodelling; and these effects could be attributed to Th2 pro-inflammatory cytokines and oxidative stress reduction as well as control of NF-kB activation.
Conclusions and implications: These results highlighted the importance of counteracting oxidative stress by flavonoids in this asthma model and suggest sakuranetin as a potential candidate for studies of treatment of asthma.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures


Similar articles
-
Chrysin alleviates allergic inflammation and airway remodeling in a murine model of chronic asthma.Int Immunopharmacol. 2016 Mar;32:24-31. doi: 10.1016/j.intimp.2016.01.005. Epub 2016 Jan 15. Int Immunopharmacol. 2016. PMID: 26780233
-
Petiveria alliacea Suppresses Airway Inflammation and Allergen-Specific Th2 Responses in Ovalbumin-Sensitized Murine Model of Asthma.Chin J Integr Med. 2018 Dec;24(12):912-919. doi: 10.1007/s11655-018-2566-5. Epub 2018 Oct 19. Chin J Integr Med. 2018. PMID: 30341485
-
The tyrosine kinase inhibitor dasatinib reduces lung inflammation and remodelling in experimental allergic asthma.Br J Pharmacol. 2016 Apr;173(7):1236-47. doi: 10.1111/bph.13430. Epub 2016 Feb 25. Br J Pharmacol. 2016. PMID: 26989986 Free PMC article.
-
Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets.Pharmacol Ther. 2018 Jan;181:169-182. doi: 10.1016/j.pharmthera.2017.08.011. Epub 2017 Aug 23. Pharmacol Ther. 2018. PMID: 28842273 Free PMC article. Review.
-
Novel concepts in airway inflammation and remodelling in asthma.Eur Respir J. 2015 Dec;46(6):1796-804. doi: 10.1183/13993003.01196-2014. Epub 2015 Nov 5. Eur Respir J. 2015. PMID: 26541520 Review.
Cited by
-
Sinomenine attenuates airway inflammation and remodeling in a mouse model of asthma.Mol Med Rep. 2016 Mar;13(3):2415-22. doi: 10.3892/mmr.2016.4816. Epub 2016 Jan 28. Mol Med Rep. 2016. PMID: 26820806 Free PMC article.
-
Airway Redox Homeostasis and Inflammation Gone Awry: From Molecular Pathogenesis to Emerging Therapeutics in Respiratory Pathology.Int J Mol Sci. 2020 Dec 7;21(23):9317. doi: 10.3390/ijms21239317. Int J Mol Sci. 2020. PMID: 33297418 Free PMC article. Review.
-
White Blood Cell Count and Serum Cytokine Profile in Tropical Hardwood Workers in Kumasi.Mediators Inflamm. 2022 Jun 27;2022:8245717. doi: 10.1155/2022/8245717. eCollection 2022. Mediators Inflamm. 2022. PMID: 35795404 Free PMC article.
-
Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0.PLoS Biol. 2020 Jul 14;18(7):e3000411. doi: 10.1371/journal.pbio.3000411. eCollection 2020 Jul. PLoS Biol. 2020. PMID: 32663221 Free PMC article.
-
Pulmonary inflammation is regulated by the levels of the vesicular acetylcholine transporter.PLoS One. 2015 Mar 27;10(3):e0120441. doi: 10.1371/journal.pone.0120441. eCollection 2015. PLoS One. 2015. PMID: 25816137 Free PMC article.
References
-
- Agrawal PK. Carbon-13 NMR of Flavonones. Amsterdan, The Netherlands: Elsevier Science Publishers; 1989.
-
- Angeli P, Prado CM, Xisto DG, Silva PL, Pássaro CP, Nakazato HD, et al. Effects of chronic L-NAME treatment lung tissue mechanics, eosinophilic and extracellular matrix responses induced by chronic pulmonary inflammation. Am J Physiol Lung Cell Mol Physiol. 2008;294:1197–1205. - PubMed
-
- Antunes MA, Abreu SC, Damaceno-Rodrigues NR, Parra ER, Capelozzi VL, Pinart M, et al. Different strains of mice present distinct lung tissue mechanics and extracellular matrix composition in a model of chronic allergic asthma. Respir Physiol Neurobiol. 2009;165:202–207. - PubMed
-
- Araujo BB, Dolhnikoff M, Silva LF, Elliot J, Lindeman JH, Ferreira DS, et al. Extracellular matrix components and regulators in the airway smooth muscle in asthma. Eur Respir J. 2008;32:61–69. - PubMed
-
- Bao ZS, Hong L, Guan Y, Dong XW, Zheng HS, Tan GL, et al. Inhibition of airway inflammation, hyperresponsiveness and remodeling by soy isoflavone in a murine model of allergic asthma. Int Immunopharmacol. 2011;11:899–906. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

