Rigorous incorporation of tautomers, ionization species, and different binding modes into ligand-based and receptor-based 3D-QSAR methods
- PMID: 23170882
- PMCID: PMC3778504
- DOI: 10.2174/1381612811319230013
Rigorous incorporation of tautomers, ionization species, and different binding modes into ligand-based and receptor-based 3D-QSAR methods
Abstract
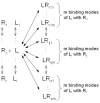
Speciation of drug candidates and receptors caused by ionization, tautomerism, and/or covalent hydration complicates ligandand receptor-based predictions of binding affinities by 3-dimensional structure-activity relationships (3D-QSAR). The speciation problem is exacerbated by tendency of tautomers to bind in multiple conformations or orientations (modes) in the same binding site. New forms of the 3D-QSAR correlation equations, capable of capturing this complexity, can be developed using the time hierarchy of all steps that lie behind the monitored biological process - binding, enzyme inhibition or receptor activity. In most cases, reversible interconversions of individual ligand and receptor species can be treated as quickly established equilibria because they are finished in a small fraction of the exposure time that is used to determine biological effects. The speciation equilibria are satisfactorily approximated by invariant fractions of individual ligand and receptor species for buffered experimental or in vivo conditions. For such situations, the observed drug-receptor association constant of a ligand is expressed as the sum of products, for each ligand and receptor species pair, of the association microconstant and the fractions of involved species. For multiple binding modes, each microconstant is expressed as the sum of microconstants of individual modes. This master equation leads to new 3D-QSAR correlation equations integrating the results of all molecular simulations or calculations, which are run for each ligand-receptor species pair separately. The multispecies, multimode 3D-QSAR approach is illustrated by a ligand-based correlation of transthyretin binding of thyroxine analogs and by a receptor-based correlation of inhibition of MK2 by benzothiophenes and pyrrolopyrimidines.
Figures
References
-
- Shimba N, Serber Z, Ledwidge R, Miller SM, Craik CS, Doetsch V. Quantitative identification of the protonation state of histidines in vitro and in vivo. Biochemistry. 2003;42:9227–34. - PubMed
-
- Clarke JA, Dawson PJ, Grigg R, Rochester CH. Spectroscopic study of the acid ionization of porphyrins. J Chem Soc Perk T. 1973;2:414–6.
-
- Braun J, Koecher M, Schlabach M, Wehrle B, Limbach HH, Vogel E. NMR study of the tautomerism of porphyrin including the kinetic HH/HD/DD isotope effects in the liquid and the solid state. J Am Chem Soc. 1994;116:6593–604.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

