Getting in shape: controlling peptide bioactivity and bioavailability using conformational constraints
- PMID: 23170954
- PMCID: PMC4847942
- DOI: 10.1021/cb300515u
Getting in shape: controlling peptide bioactivity and bioavailability using conformational constraints
Abstract
Chemical biologists commonly seek out correlations between the physicochemical properties of molecules and their behavior in biological systems. However, a new paradigm is emerging for peptides in which conformation is recognized as the primary determinant of bioactivity and bioavailability. This review highlights an emerging body of work that directly addresses how a peptide's conformation controls its biological effects, cell penetration, and intestinal absorption. Based on this work, the dream of mimicking the potency and bioavailability of natural product peptides is getting closer to reality.
Figures

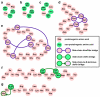
References
-
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 1997;23:3–25. - PubMed
-
- Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002;45:2615–2623. - PubMed
-
- Driggers EM, Hale SP, Lee J, Terrett NK. The exploration of macrocycles for drug discovery – an underexploited structural class. Nat. Rev. Drug Discovery. 2008;7:608–624. - PubMed
-
- Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS. Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988;31:2235–2246. - PubMed
-
- Duarte CD, Barreiro EJ, Fraga CAM. Privileged structures: A useful concept for the rational design of new lead drug candidates. Mini-Rev. Med. Chem. 2007;7:1108–1119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

