Properties of membrane-incorporated WALP peptides that are anchored on only one end
- PMID: 23171005
- PMCID: PMC3527101
- DOI: 10.1021/bi301394z
Properties of membrane-incorporated WALP peptides that are anchored on only one end
Abstract
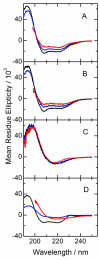
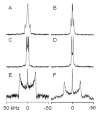
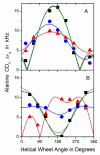
Peptides of the "WALP" family, acetyl-GWW(LA)(n)LWWA-[ethanol]amide, have proven to be opportune models for investigating lipid-peptide interactions. Because the average orientations and motional behavior of the N- and C-terminal Trp (W) residues differ, it is of interest to investigate how the positions of the tryptophans influence the properties of the membrane-incorporated peptides. To address this question, we synthesized acetyl-GGWW(LA)(n)-ethanolamide and acetyl-(AL)(n)WWG-ethanolamide, in which n = 4 or 8, which we designate as "N-anchored" and "C-anchored" peptides, respectively. Selected (2)H or (15)N labels were incorporated for solid-state nuclear magnetic resonance (NMR) spectroscopy. These peptides can be considered "half"-anchored WALP peptides, having only one pair of interfacial Trp residues near either the amino or the carboxyl terminus. The hydrophobic lengths of the (n = 8) peptides are similar to that of WALP23. These longer half-anchored WALP peptides incorporate into lipid bilayers as α-helices, as reflected in their circular dichroism spectra. Solid-state NMR experiments indicate that the longer peptide helices assume defined transmembrane orientations with small non-zero average tilt angles and moderate to high dynamic averaging in bilayer membranes of 1,2-dioleoylphosphatidylcholine, 1,2-dimyristoylphosphatidylcholine, and 1,2-dilauroylphosphatidylcholine. The intrinsically small apparent tilt angles suggest that interactions of aromatic residues with lipid headgroups may play an important role in determining the magnitude of the peptide tilt in the bilayer membrane. The shorter (n = 4) peptides, in stark contrast to the longer peptides, display NMR spectra that are characteristic of greatly reduced motional averaging, probably because of peptide aggregation in the bilayer environment, and CD spectra that are characteristic of β-structure.
Figures




Similar articles
-
Tyrosine replacing tryptophan as an anchor in GWALP peptides.Biochemistry. 2012 Mar 13;51(10):2044-53. doi: 10.1021/bi201732e. Epub 2012 Mar 5. Biochemistry. 2012. PMID: 22364236 Free PMC article.
-
Charged or aromatic anchor residue dependence of transmembrane peptide tilt.J Biol Chem. 2010 Oct 8;285(41):31723-30. doi: 10.1074/jbc.M110.152470. Epub 2010 Jul 28. J Biol Chem. 2010. PMID: 20667827 Free PMC article.
-
Orientation and motion of tryptophan interfacial anchors in membrane-spanning peptides.Biochemistry. 2007 Jun 26;46(25):7514-24. doi: 10.1021/bi700082v. Epub 2007 May 27. Biochemistry. 2007. PMID: 17530863 Free PMC article.
-
Helical distortion in tryptophan- and lysine-anchored membrane-spanning alpha-helices as a function of hydrophobic mismatch: a solid-state deuterium NMR investigation using the geometric analysis of labeled alanines method.Biophys J. 2008 Jan 15;94(2):480-91. doi: 10.1529/biophysj.106.097543. Epub 2007 Sep 7. Biophys J. 2008. PMID: 17827234 Free PMC article.
-
Lipid bilayers: an essential environment for the understanding of membrane proteins.Magn Reson Chem. 2007 Dec;45 Suppl 1:S2-11. doi: 10.1002/mrc.2077. Epub 2007 Dec 19. Magn Reson Chem. 2007. PMID: 18095258 Review.
Cited by
-
Transmembrane Helix Integrity versus Fraying To Expose Hydrogen Bonds at a Membrane-Water Interface.Biochemistry. 2019 Feb 12;58(6):633-645. doi: 10.1021/acs.biochem.8b01119. Epub 2019 Jan 3. Biochemistry. 2019. PMID: 30565458 Free PMC article.
-
Kinetics of peptide folding in lipid membranes.Biopolymers. 2015 Jul;104(4):281-90. doi: 10.1002/bip.22640. Biopolymers. 2015. PMID: 25808575 Free PMC article. Review.
-
Influence of High pH and Cholesterol on Single Arginine-Containing Transmembrane Peptide Helices.Biochemistry. 2016 Nov 15;55(45):6337-6343. doi: 10.1021/acs.biochem.6b00896. Epub 2016 Nov 4. Biochemistry. 2016. PMID: 27782382 Free PMC article.
-
A novel pH-dependent membrane peptide that binds to EphA2 and inhibits cell migration.Elife. 2018 Sep 17;7:e36645. doi: 10.7554/eLife.36645. Elife. 2018. PMID: 30222105 Free PMC article.
-
Peptide-lipid interactions: experiments and applications.Int J Mol Sci. 2013 Sep 12;14(9):18758-89. doi: 10.3390/ijms140918758. Int J Mol Sci. 2013. PMID: 24036440 Free PMC article.
References
-
- Killian JA, Salemink I, dePlanque MRR, Lindblom G, Koeppe RE, II, Greathouse DV. Induction of nonbilayer structures in diacylphosphatidylcholine model membranes by transmembrane alpha-helical peptides: Importance of hydrophobic mismatch and proposed role of tryptophans. Biochemistry. 1996;35:1037–1045. - PubMed
-
- Schiffer M, Chang CH, Stevens FJ. The functions of tryptophan residues in membrane proteins. Protein Eng. 1992;5:213–214. - PubMed
-
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. - PubMed
-
- de Planque MR, Boots JW, Rijkers DT, Liskamp RM, Greathouse DV, Killian JA. The effects of hydrophobic mismatch between phosphatidylcholine bilayers and transmembrane alpha-helical peptides depend on the nature of interfacially exposed aromatic and charged residues. Biochemistry. 2002;41:8396–8404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

