Review: experimental manipulations of microglia in mouse models of Alzheimer's pathology: activation reduces amyloid but hastens tau pathology
- PMID: 23171029
- PMCID: PMC4300851
- DOI: 10.1111/nan.12002
Review: experimental manipulations of microglia in mouse models of Alzheimer's pathology: activation reduces amyloid but hastens tau pathology
Abstract
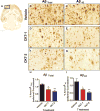
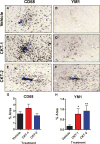
The inflammation hypothesis of Alzheimer's pathogenesis has directed much scientific effort towards ameliorating this disease. The development of mouse models of amyloid deposition permitted direct tests of the proposal that amyloid-activated microglia could cause neurodegeneration in vivo. Many approaches to manipulating microglial activation have been applied to these mouse models, and are the subject of this review. In general, these results do not support a direct neuricidal action of microglia in mouse amyloid models under any activation state. Some of the manipulations cause both a reduction in pathology and a reduction in microglial activation. However, at least for agents like ibuprofen, this outcome may result from a direct action on amyloid production, and a reduction in the microglial-provoking amyloid deposits, rather than from reduced microglial activation leading to a decline in amyloid deposition. Instead, a surprising number of the experimental manipulations which increase microglial activation lead to enhanced clearance of the amyloid deposits. Both the literature and new data presented here suggest that either classical or alternative activation of microglia can lead to enhanced amyloid clearance. However, a limited number of studies comparing the same treatments in amyloid-depositing vs. tau-depositing mice find the opposite effects. Treatments that benefit amyloid pathology accelerate tau pathology. This observation argues strongly that potential treatments be tested for impact on both amyloid and tau pathology before consideration of testing in humans.
© 2012 British Neuropathological Society.
Figures
References
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. NeurobiolAging. 2000;21:383–421. - PMC - PubMed
-
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. NeurosciLett. 1987;79:195–200. - PubMed
-
- Eikelenboom P, Stam FC. Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol (Berl) 1982;57:239–42. - PubMed
-
- Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. NeurobiolAging. 1988;9:339–49. - PubMed
-
- Breitner JC, Welsh KA, Helms MJ, Gaskell PC, Gau BA, Roses AD, Pericak-Vance MA, Saunders AM. Delayed onset of Alzheimer’s disease with nonsteroidal anti-inflammatory and histamine H2 blocking drugs. NeurobiolAging. 1995;16:523–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

