Transcriptional and metabolomic analysis of Ascophyllum nodosum mediated freezing tolerance in Arabidopsis thaliana
- PMID: 23171218
- PMCID: PMC3560180
- DOI: 10.1186/1471-2164-13-643
Transcriptional and metabolomic analysis of Ascophyllum nodosum mediated freezing tolerance in Arabidopsis thaliana
Abstract
Background: We have previously shown that lipophilic components (LPC) of the brown seaweed Ascophyllum nodosum (ANE) improved freezing tolerance in Arabidopsis thaliana. However, the mechanism(s) of this induced freezing stress tolerance is largely unknown. Here, we investigated LPC induced changes in the transcriptome and metabolome of A. thaliana undergoing freezing stress.
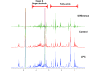
Results: Gene expression studies revealed that the accumulation of proline was mediated by an increase in the expression of the proline synthesis genes P5CS1 and P5CS2 and a marginal reduction in the expression of the proline dehydrogenase (ProDH) gene. Moreover, LPC application significantly increased the concentration of total soluble sugars in the cytosol in response to freezing stress. Arabidopsis sfr4 mutant plants, defective in the accumulation of free sugars, treated with LPC, exhibited freezing sensitivity similar to that of untreated controls. The 1H NMR metabolite profile of LPC-treated Arabidopsis plants exposed to freezing stress revealed a spectrum dominated by chemical shifts (δ) representing soluble sugars, sugar alcohols, organic acids and lipophilic components like fatty acids, as compared to control plants. Additionally, 2D NMR spectra suggested an increase in the degree of unsaturation of fatty acids in LPC treated plants under freezing stress. These results were supported by global transcriptome analysis. Transcriptome analysis revealed that LPC treatment altered the expression of 1113 genes (5%) in comparison with untreated plants. A total of 463 genes (2%) were up regulated while 650 genes (3%) were down regulated.
Conclusion: Taken together, the results of the experiments presented in this paper provide evidence to support LPC mediated freezing tolerance enhancement through a combination of the priming of plants for the increased accumulation of osmoprotectants and alteration of cellular fatty acid composition.
Figures






References
-
- Bray EA, Bailey-Serres J, Weretilnyk E. In: Biochemistry and Molecular Biology of Plants. Gruissem W, Buchannan B, Jones R, editor. Rockville: American Society of Plant Biologists; 2000. Responses to abiotic stresses; pp. 1158–1249.
-
- Fuller MP, Hamed F, Wisniewski M, Glenn DM. Protection of plants from frost using hydrophobic particle film and acrylic polymer. Ann Appl Biol. 2003;143:97–97.
-
- Ilker R, Warring AJ, Lyons JM, Breidenbach RW. The cytological responses of tomato seedling cotyledons to chilling and the influence of membrane modifications upon these responses. Protoplasma. 1976;90:229–252. doi: 10.1007/BF01275677. - DOI
-
- Temple WD, Bomke AA. Effects of Kelp (Macrocystis integrifolia and Eklonia maxima) foliar applications on bean crop growth. Plant Soil. 1989;117:85–92. doi: 10.1007/BF02206260. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

