Differential susceptibility of inbred mouse strains to chlorine-induced airway fibrosis
- PMID: 23171502
- PMCID: PMC3543638
- DOI: 10.1152/ajplung.00272.2012
Differential susceptibility of inbred mouse strains to chlorine-induced airway fibrosis
Abstract
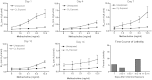
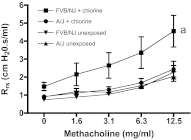
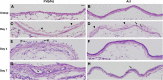
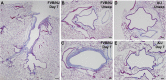
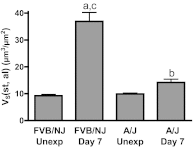
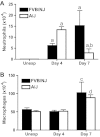
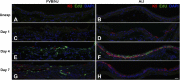
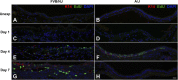
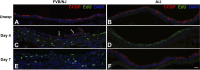
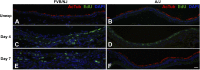
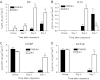
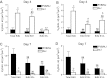
Chlorine is a reactive gas that is considered a chemical threat agent. Humans who develop acute lung injury from chlorine inhalation typically recover normal lung function; however, a subset can experience chronic airway disease. To examine pathological changes following chlorine-induced lung injury, mice were exposed to a single high dose of chlorine, and repair of the lung was analyzed at multiple times after exposure. In FVB/NJ mice, chlorine inhalation caused pronounced fibrosis of larger airways that developed by day 7 after exposure and was associated with airway hyperreactivity. In contrast, A/J mice had little or no airway fibrosis and had normal lung function at day 7. Unexposed FVB/NJ mice had less keratin 5 staining (basal cell marker) than A/J mice in large intrapulmonary airways where epithelial repair was poor and fibrosis developed after chlorine exposure. FVB/NJ mice had large areas devoid of epithelium on day 1 after exposure leading to fibroproliferative lesions on days 4 and 7. A/J mice had airways covered by squamous keratin 5-stained cells on day 1 that transitioned to a highly proliferative reparative epithelium by day 4 followed by the reappearance of ciliated and Clara cells by day 7. The data suggest that lack of basal cells in the large intrapulmonary airways and failure to effect epithelial repair at these sites are factors contributing to the development of airway fibrosis in FVB/NJ mice. The observed differences in susceptibility to chlorine-induced airway disease provide a model in which mechanisms and treatment of airway fibrosis can be investigated.
Figures

Similar articles
-
Inhibition of chlorine-induced airway fibrosis by budesonide.Toxicol Appl Pharmacol. 2019 Jan 15;363:11-21. doi: 10.1016/j.taap.2018.08.024. Epub 2018 Sep 3. Toxicol Appl Pharmacol. 2019. PMID: 30189237 Free PMC article.
-
Acute lung injury induced by chlorine inhalation in C57BL/6 and FVB/N mice.Inhal Toxicol. 2008 Jul;20(9):783-93. doi: 10.1080/08958370802007841. Inhal Toxicol. 2008. PMID: 18645717
-
Abnormal epithelial structure and chronic lung inflammation after repair of chlorine-induced airway injury.Am J Physiol Lung Cell Mol Physiol. 2015 Jan 15;308(2):L168-78. doi: 10.1152/ajplung.00226.2014. Epub 2014 Nov 14. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25398987 Free PMC article.
-
Persistent effects of chlorine inhalation on respiratory health.Ann N Y Acad Sci. 2016 Aug;1378(1):33-40. doi: 10.1111/nyas.13139. Epub 2016 Jul 6. Ann N Y Acad Sci. 2016. PMID: 27385061 Free PMC article. Review.
-
Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models.Proc Am Thorac Soc. 2010 Jul;7(4):257-63. doi: 10.1513/pats.201001-008SM. Proc Am Thorac Soc. 2010. PMID: 20601629 Free PMC article. Review.
Cited by
-
Heme Attenuation Ameliorates Irritant Gas Inhalation-Induced Acute Lung Injury.Antioxid Redox Signal. 2016 Jan 10;24(2):99-112. doi: 10.1089/ars.2015.6347. Epub 2015 Dec 14. Antioxid Redox Signal. 2016. PMID: 26376667 Free PMC article.
-
Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury.Am J Physiol Lung Cell Mol Physiol. 2015 May 1;308(9):L891-903. doi: 10.1152/ajplung.00377.2014. Epub 2015 Mar 6. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25747964 Free PMC article.
-
Inhibition of chlorine-induced airway fibrosis by budesonide.Toxicol Appl Pharmacol. 2019 Jan 15;363:11-21. doi: 10.1016/j.taap.2018.08.024. Epub 2018 Sep 3. Toxicol Appl Pharmacol. 2019. PMID: 30189237 Free PMC article.
-
Halogen Inhalation-Induced Lung Injury and Acute Respiratory Distress Syndrome.Chin Med J (Engl). 2018 May 20;131(10):1214-1219. doi: 10.4103/0366-6999.231515. Chin Med J (Engl). 2018. PMID: 29722341 Free PMC article. Review.
-
Effects of metal nanoparticles on tight junction-associated proteins via HIF-1α/miR-29b/MMPs pathway in human epidermal keratinocytes.Part Fibre Toxicol. 2021 Mar 19;18(1):13. doi: 10.1186/s12989-021-00405-2. Part Fibre Toxicol. 2021. PMID: 33740985 Free PMC article.
References
-
- Boulet LP, Laviolette M, Turcotte H, Cartier A, Dugas M, Malo JL, Boutet M. Bronchial subepithelial fibrosis correlates with airway responsiveness to methacholine. Chest 112: 45–52, 1997 - PubMed
-
- Brooks SM, Weiss MA, Bernstein IL. Reactive airways dysfunction syndrome (RADS). Persistent asthma syndrome after high level irritant exposures. Chest 88: 376–384, 1985 - PubMed
-
- Caniggia I, Tseu I, Rolland G, Edelson J, Tanswell AK, Post M. Inhibition of fibroblast growth by epithelial cells in fetal rat lung. Am J Respir Cell Mol Biol 13: 91–98, 1995 - PubMed
-
- Chang-Yeung M, Lam S, Kennedy SM, Frew AJ. Persistent asthma after repeated exposure to high concentrations of gases in pulpmills. Am J Respir Crit Care Med 149: 1676–1680, 1994 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

