Myelin is dependent on the Charcot-Marie-Tooth Type 4H disease culprit protein FRABIN/FGD4 in Schwann cells
- PMID: 23171661
- PMCID: PMC3525053
- DOI: 10.1093/brain/aws275
Myelin is dependent on the Charcot-Marie-Tooth Type 4H disease culprit protein FRABIN/FGD4 in Schwann cells
Abstract
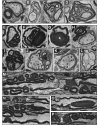
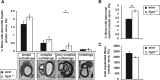
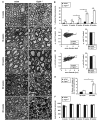
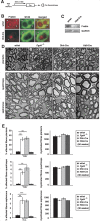
Studying the function and malfunction of genes and proteins associated with inherited forms of peripheral neuropathies has provided multiple clues to our understanding of myelinated nerves in health and disease. Here, we have generated a mouse model for the peripheral neuropathy Charcot-Marie-Tooth disease type 4H by constitutively disrupting the mouse orthologue of the suspected culprit gene FGD4 that encodes the small RhoGTPase Cdc42-guanine nucleotide exchange factor Frabin. Lack of Frabin/Fgd4 causes dysmyelination in mice in early peripheral nerve development, followed by profound myelin abnormalities and demyelination at later stages. At the age of 60 weeks, this was accompanied by electrophysiological deficits. By crossing mice carrying alleles of Frabin/Fgd4 flanked by loxP sequences with animals expressing Cre recombinase in a cell type-specific manner, we show that Schwann cell-autonomous Frabin/Fgd4 function is essential for proper myelination without detectable primary contributions from neurons. Deletion of Frabin/Fgd4 in Schwann cells of fully myelinated nerve fibres revealed that this protein is not only required for correct nerve development but also for accurate myelin maintenance. Moreover, we established that correct activation of Cdc42 is dependent on Frabin/Fgd4 function in healthy peripheral nerves. Genetic disruption of Cdc42 in Schwann cells of adult myelinated nerves resulted in myelin alterations similar to those observed in Frabin/Fgd4-deficient mice, indicating that Cdc42 and the Frabin/Fgd4-Cdc42 axis are critical for myelin homeostasis. In line with known regulatory roles of Cdc42, we found that Frabin/Fgd4 regulates Schwann cell endocytosis, a process that is increasingly recognized as a relevant mechanism in peripheral nerve pathophysiology. Taken together, our results indicate that regulation of Cdc42 by Frabin/Fgd4 in Schwann cells is critical for the structure and function of the peripheral nervous system. In particular, this regulatory link is continuously required in adult fully myelinated nerve fibres. Thus, mechanisms regulated by Frabin/Fgd4-Cdc42 are promising targets that can help to identify additional regulators of myelin development and homeostasis, which may crucially contribute also to malfunctions in different types of peripheral neuropathies.
Figures




References
-
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–74. - PubMed
-
- Azzedine H, Bolino A, Taieb T, Birouk N, Di Duca M, Bouhouche A, et al. Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma. Am J Hum Genet. 2003;72:1141–53. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

