Periadolescent maturation of the prefrontal cortex is sex-specific and is disrupted by prenatal stress
- PMID: 23172080
- PMCID: PMC4479145
- DOI: 10.1002/cne.23262
Periadolescent maturation of the prefrontal cortex is sex-specific and is disrupted by prenatal stress
Abstract
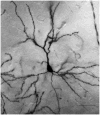
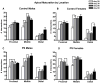
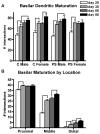
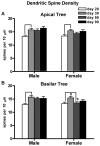
The prefrontal cortex (PFC) undergoes dramatic, sex-specific maturation during adolescence. Adolescence is a vulnerable window for developing mental illnesses that show significant sexual dimorphisms. Gestational stress is associated with increased risk for both schizophrenia, which is more common among men, and cognitive deficits. We have shown that male, but not female, rats exposed to prenatal stress develop postpubertal deficits in cognitive behaviors supported by the prefrontal cortex. Here we tested the hypothesis that repeated variable prenatal stress during the third week of rat gestation disrupts periadolescent development of prefrontal neurons in a sex-specific fashion. Using Golgi-Cox stained tissue, we compared dendritic arborization and spine density of prelimbic layer III neurons in prenatally stressed and control animals at juvenile (day 20), prepubertal (day 30), postpubertal (day 56), and adult (day 90) ages (N = 115). Dendritic ramification followed a sex-specific pattern that was disrupted during adolescence in prenatally stressed males, but not in females. In contrast, the impact of prenatal stress on the female PFC was not evident until adulthood. Prenatal stress also caused reductions in brain and body weights, and the latter effect was more pronounced among males. Additionally, there was a trend toward reduced testosterone levels for adult prenatally stressed males. Our findings indicate that, similarly to humans, the rat PFC undergoes sex-specific development during adolescence and furthermore that this process is disrupted by prenatal stress. These findings may be relevant to both the development of normal sex differences in cognition as well as differential male-female vulnerability to psychiatric conditions.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

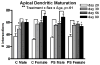
Similar articles
-
Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy.Eur J Neurosci. 2006 Sep;24(5):1477-87. doi: 10.1111/j.1460-9568.2006.05024.x. Epub 2006 Sep 8. Eur J Neurosci. 2006. PMID: 16965544
-
Gender- and anxiety level-dependent effects of perinatal stress exposure on medial prefrontal cortex.Exp Neurol. 2016 Jan;275 Pt 2:274-84. doi: 10.1016/j.expneurol.2015.06.005. Epub 2015 Jun 6. Exp Neurol. 2016. PMID: 26057948
-
Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring.Synapse. 2009 Sep;63(9):794-804. doi: 10.1002/syn.20664. Synapse. 2009. PMID: 19489049
-
Sex-dependent changes induced by prenatal stress in cortical and hippocampal morphology and behaviour in rats: an update.Stress. 2011 Nov;14(6):604-13. doi: 10.3109/10253890.2011.588294. Epub 2011 Jul 26. Stress. 2011. PMID: 21790452 Review.
-
Schizophrenia: a tale of two critical periods for prefrontal cortical development.Transl Psychiatry. 2015 Aug 18;5(8):e623. doi: 10.1038/tp.2015.115. Transl Psychiatry. 2015. PMID: 26285133 Free PMC article. Review.
Cited by
-
Functional and structural remodeling of glutamate synapses in prefrontal and frontal cortex induced by behavioral stress.Front Psychiatry. 2015 Apr 27;6:60. doi: 10.3389/fpsyt.2015.00060. eCollection 2015. Front Psychiatry. 2015. PMID: 25964763 Free PMC article. Review.
-
Mini-review: Elucidating the psychological, physical, and sex-based interactions between HIV infection and stress.Neurosci Lett. 2021 Mar 16;747:135698. doi: 10.1016/j.neulet.2021.135698. Epub 2021 Feb 1. Neurosci Lett. 2021. PMID: 33540057 Free PMC article. Review.
-
The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course.Neuron. 2013 Jul 10;79(1):16-29. doi: 10.1016/j.neuron.2013.06.028. Neuron. 2013. PMID: 23849196 Free PMC article. Review.
-
Multivariate synaptic and behavioral profiling reveals new developmental endophenotypes in the prefrontal cortex.Sci Rep. 2016 Oct 21;6:35504. doi: 10.1038/srep35504. Sci Rep. 2016. PMID: 27765946 Free PMC article.
-
Sex differences in behavior and neural development and their role in adolescent vulnerability to substance use.Behav Brain Res. 2016 Feb 1;298(Pt A):15-26. doi: 10.1016/j.bbr.2015.04.008. Epub 2015 Apr 13. Behav Brain Res. 2016. PMID: 25882721 Free PMC article. Review.
References
-
- Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Archives of general psychiatry. 2003;60(6):565–571. - PubMed
-
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and biobehavioral reviews. 2003;27(1–2):3–18. - PubMed
-
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse (New York, NY. 2000;37(2):167–169. - PubMed
-
- Anderson DK, Rhees RW, Fleming DE. Effects of prenatal stress on differentiation of the sexually dimorphic nucleus of the preoptic area (SDN-POA) of the rat brain. Brain Res. 1985;332(1):113–118. - PubMed
-
- Anderson RH, Fleming DE, Rhees RW, Kinghorn E. Relationships between sexual activity, plasma testosterone, and the volume of the sexually dimorphic nucleus of the preoptic area in prenatally stressed and non-stressed rats. Brain Res. 1986;370(1):1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

