A placebo-controlled, randomized trial of mesenchymal stem cells in COPD
- PMID: 23172272
- PMCID: PMC4694112
- DOI: 10.1378/chest.12-2094
A placebo-controlled, randomized trial of mesenchymal stem cells in COPD
Abstract
Background: COPD is a devastating disease affecting millions worldwide. As disease pathogenesis includes both chronic pulmonary and systemic inflammation, antiinflammatory effects of systemically administered mesenchymal stem cells (MSCs) may decrease inflammation, resulting in improved lung function and quality of life. The goal of this study was to assess safety and to perform an initial evaluation of the potential efficacy of systemic MSC administration to patients with moderate to severe COPD.
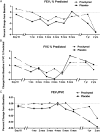
Methods: Sixty-two patients at six sites were randomized to double-blinded IV infusions of either allogeneic MSCs (Prochymal; Osiris Therapeutics Inc) or vehicle control. Patients received four monthly infusions (100 × 10⁶ cells/infusion) and were subsequently followed for 2 years after the first infusion. End points included comprehensive safety evaluation, pulmonary function testing (PFT), and quality-of-life indicators including questionnaires, 6MWT, and assessments of systemic inflammation.
Results: All study patients completed the full infusion protocol, and 74% completed the 2-year follow-up. There were no infusional toxicities and no deaths or serious adverse events deemed related to MSC administration. There were no significant differences in the overall number of adverse events, frequency of COPD exacerbations, or worsening of disease in patients treated with MSCs. There were no significant differences in PFTs or quality-of-life indicators; however, an early, significant decrease in levels of circulating C-reactive protein (CRP) was observed in patients treated with MSCs who had elevated CRP levels at study entry.
Conclusions: Systemic MSC administration appears to be safe in patients with moderate to severe COPD and provides a basis for subsequent cell therapy investigations.
Trial registry: ClinicalTrials.gov; No.: NCT00683722; URL: www.clinicaltrials.gov.
Figures


Comment in
-
Cell therapy for lung disease: a step forward.Chest. 2013 Jun;143(6):1525-1527. doi: 10.1378/chest.12-2993. Chest. 2013. PMID: 23732575 No abstract available.
Similar articles
-
Effect of mesenchymal stromal cell infusions on lung function in COPD patients with high CRP levels.Respir Res. 2021 May 8;22(1):142. doi: 10.1186/s12931-021-01734-8. Respir Res. 2021. PMID: 33964910 Free PMC article. Clinical Trial.
-
Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study.Stem Cell Res Ther. 2020 Feb 13;11(1):60. doi: 10.1186/s13287-020-1583-4. Stem Cell Res Ther. 2020. PMID: 32054512 Free PMC article.
-
Combined Bone Marrow-Derived Mesenchymal Stromal Cell Therapy and One-Way Endobronchial Valve Placement in Patients with Pulmonary Emphysema: A Phase I Clinical Trial.Stem Cells Transl Med. 2017 Mar;6(3):962-969. doi: 10.1002/sctm.16-0315. Epub 2016 Dec 9. Stem Cells Transl Med. 2017. PMID: 28186686 Free PMC article. Clinical Trial.
-
Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial.Lancet Respir Med. 2019 Feb;7(2):154-162. doi: 10.1016/S2213-2600(18)30418-1. Epub 2018 Nov 16. Lancet Respir Med. 2019. PMID: 30455077 Free PMC article. Clinical Trial.
-
Stem Cell Therapy for COPD: Hope and Exploitation.Chest. 2021 Oct;160(4):1271-1281. doi: 10.1016/j.chest.2021.04.020. Epub 2021 Apr 21. Chest. 2021. PMID: 33894254 Review.
Cited by
-
Effect of mesenchymal stromal cell infusions on lung function in COPD patients with high CRP levels.Respir Res. 2021 May 8;22(1):142. doi: 10.1186/s12931-021-01734-8. Respir Res. 2021. PMID: 33964910 Free PMC article. Clinical Trial.
-
Secretome of Multipotent Mesenchymal Stromal Cells as a Promising Treatment and for Rehabilitation of Patients with the Novel Coronaviral Infection.Her Russ Acad Sci. 2021;91(2):170-175. doi: 10.1134/S101933162102012X. Epub 2021 Jun 10. Her Russ Acad Sci. 2021. PMID: 34131372 Free PMC article.
-
The Therapeutic Effects of Optimal Dose of Mesenchymal Stem Cells in a Murine Model of an Elastase Induced-Emphysema.Tuberc Respir Dis (Seoul). 2015 Jul;78(3):239-45. doi: 10.4046/trd.2015.78.3.239. Epub 2015 Jun 30. Tuberc Respir Dis (Seoul). 2015. PMID: 26175778 Free PMC article.
-
Mesenchymal Stromal Cells to Regenerate Emphysema: On the Horizon?Respiration. 2018;96(2):148-158. doi: 10.1159/000488149. Epub 2018 May 2. Respiration. 2018. PMID: 29719298 Free PMC article. Review.
-
A phase I study for intravenous autologous mesenchymal stromal cell administration to patients with severe emphysema.QJM. 2016 May;109(5):331-6. doi: 10.1093/qjmed/hcw001. Epub 2016 Jan 27. QJM. 2016. PMID: 26819296 Free PMC article. Clinical Trial.
References
-
- Miniño AM, Xu JQ, Kochanek K. Deaths: preliminary data for 2008. Natl Vital Stat Rep. 2010;59(2). - PubMed
-
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27(2):397-412. - PubMed
-
- Eisner MD, Anthonisen N, Coultas D, et al. ; Committee on Nonsmoking COPD, Environmental and Occupational Health Assembly. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(5):693-718. - PubMed
-
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267-274. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

