Importance of the pluripotency factor LIN28 in the mammalian nucleolus during early embryonic development
- PMID: 23172912
- PMCID: PMC3912245
- DOI: 10.1242/dev.083279
Importance of the pluripotency factor LIN28 in the mammalian nucleolus during early embryonic development
Abstract
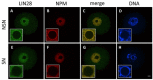
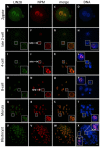
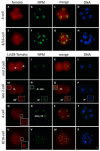
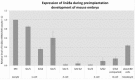
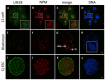
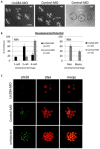
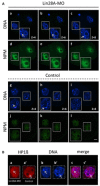
The maternal nucleolus is required for proper activation of the embryonic genome (EGA) and early embryonic development. Nucleologenesis is characterized by the transformation of a nucleolar precursor body (NPB) to a mature nucleolus during preimplantation development. However, the function of NPBs and the involved molecular factors are unknown. We uncover a novel role for the pluripotency factor LIN28, the biological significance of which was previously demonstrated in the reprogramming of human somatic cells to induced pluripotent stem (iPS) cells. Here, we show that LIN28 accumulates at the NPB and the mature nucleolus in mouse preimplantation embryos and embryonic stem cells (ESCs), where it colocalizes with the nucleolar marker B23 (nucleophosmin 1). LIN28 has nucleolar localization in non-human primate (NHP) preimplantation embryos, but is cytoplasmic in NHP ESCs. Lin28 transcripts show a striking decline before mouse EGA, whereas LIN28 protein localizes to NPBs at the time of EGA. Following knockdown with a Lin28 morpholino, the majority of embryos arrest between the 2- and 4-cell stages and never develop to morula or blastocyst. Lin28 morpholino-injected embryos arrested at the 2-cell stage were not enriched with nucleophosmin at presumptive NPB sites, indicating that functional NPBs were not assembled. Based on these results, we propose that LIN28 is an essential factor of nucleologenesis during early embryonic development.
Figures
Similar articles
-
The nature of the 'nucleolus precursor body' in early preimplantation embryos: a review of fine-structure cytochemical, immunocytochemical and autoradiographic data related to nucleolar function.Zygote. 1998 May;6(2):183-91. doi: 10.1017/s0967199498000112. Zygote. 1998. PMID: 9770784 Review.
-
Nucleolar re-activation is delayed in mouse embryos cloned from two different cell lines.Mol Reprod Dev. 2009 Feb;76(2):132-41. doi: 10.1002/mrd.20936. Mol Reprod Dev. 2009. PMID: 18470874
-
Nucleolus precursor body (NPB): a distinct structure in mammalian oocytes and zygotes.Nucleus. 2014;5(6):493-8. doi: 10.4161/19491034.2014.990858. Nucleus. 2014. PMID: 25495074 Free PMC article. Review.
-
LIN28 coordinately promotes nucleolar/ribosomal functions and represses the 2C-like transcriptional program in pluripotent stem cells.Protein Cell. 2022 Jul;13(7):490-512. doi: 10.1007/s13238-021-00864-5. Epub 2021 Jul 31. Protein Cell. 2022. PMID: 34331666 Free PMC article.
-
The nucleolar protein NOP2 is required for nucleolar maturation and ribosome biogenesis during preimplantation development in mammals.FASEB J. 2020 Feb;34(2):2715-2729. doi: 10.1096/fj.201902623R. Epub 2019 Dec 24. FASEB J. 2020. PMID: 31908012
Cited by
-
Liver cancer initiation requires translational activation by an oncofetal regulon involving LIN28 proteins.J Clin Invest. 2024 Jun 13;134(15):e165734. doi: 10.1172/JCI165734. J Clin Invest. 2024. PMID: 38875287 Free PMC article.
-
Efficient generation of endogenous protein reporters for mouse development.Development. 2021 Jul 1;148(13):dev197418. doi: 10.1242/dev.197418. Epub 2021 Jun 29. Development. 2021. PMID: 34036333 Free PMC article.
-
Tumor suppressor let-7 acts as a key regulator for pluripotency gene expression in Muse cells.Cell Mol Life Sci. 2024 Jan 23;81(1):54. doi: 10.1007/s00018-023-05089-9. Cell Mol Life Sci. 2024. PMID: 38261036 Free PMC article.
-
Upregulating Lin28a Promotes Axon Regeneration in Adult Mice with Optic Nerve and Spinal Cord Injury.Mol Ther. 2020 Aug 5;28(8):1902-1917. doi: 10.1016/j.ymthe.2020.04.010. Epub 2020 Apr 15. Mol Ther. 2020. PMID: 32353321 Free PMC article.
-
Diverse Regulators of Human Ribosome Biogenesis Discovered by Changes in Nucleolar Number.Cell Rep. 2018 Feb 13;22(7):1923-1934. doi: 10.1016/j.celrep.2018.01.056. Cell Rep. 2018. PMID: 29444442 Free PMC article.
References
-
- Balzer E., Moss E. G. (2007). Localization of the developmental timing regulator Lin28 to mRNP complexes, P-bodies and stress granules. RNA Biol. 4, 16–25 - PubMed
-
- Boisvert F. M., van Koningsbruggen S., Navascués J., Lamond A. I. (2007). The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 8, 574–585 - PubMed
-
- Darr H., Benvenisty N. (2009). Genetic analysis of the role of the reprogramming gene LIN-28 in human embryonic stem cells. Stem Cells 27, 352–362 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

