Macrophage migration inhibitory factor acts as a neurotrophin in the developing inner ear
- PMID: 23172918
- PMCID: PMC3509728
- DOI: 10.1242/dev.066647
Macrophage migration inhibitory factor acts as a neurotrophin in the developing inner ear
Erratum in
- Development. 2013 Jan 15;140(2):479. Koch, Alicia E [corrected to Koch, Alisa E]
Abstract
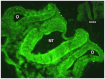
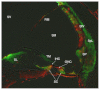
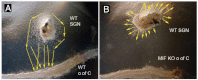
This study is the first to demonstrate that macrophage migration inhibitory factor (MIF), an immune system 'inflammatory' cytokine that is released by the developing otocyst, plays a role in regulating early innervation of the mouse and chick inner ear. We demonstrate that MIF is a major bioactive component of the previously uncharacterized otocyst-derived factor, which directs initial neurite outgrowth from the statoacoustic ganglion (SAG) to the developing inner ear. Recombinant MIF acts as a neurotrophin in promoting both SAG directional neurite outgrowth and neuronal survival and is expressed in both the developing and mature inner ear of chick and mouse. A MIF receptor, CD74, is found on both embryonic SAG neurons and adult mouse spiral ganglion neurons. Mif knockout mice are hearing impaired and demonstrate altered innervation to the organ of Corti, as well as fewer sensory hair cells. Furthermore, mouse embryonic stem cells become neuron-like when exposed to picomolar levels of MIF, suggesting the general importance of this cytokine in neural development.
Figures


References
-
- Armstrong B. D., Hu Z., Abad C., Yamamoto M., Rodriguez W. I., Cheng J., Tam J., Gomariz R. P., Patterson P. H., Waschek J. A. (2003). Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J. Neurosci. Res. 74, 240–247 - PubMed
-
- Bajetto A., Bonavia R., Barbero S., Florio T., Schettini G. (2001). Chemokines and their receptors in the central nervous system. Front. Neuroendocrinol. 22, 147–184 - PubMed
-
- Barald K. F., Kelley M. W. (2004). From placode to polarization: new tunes in inner ear development. Development 131, 4119–4130 - PubMed
-
- Barald K. F., Lindberg K. H., Hardiman K., Kavka A. I., Lewis J. E., Victor J. C., Gardner C. A., Poniatowski A. (1997). Immortalized cell lines from embryonic avian and murine otocysts: tools for molecular studies of the developing inner ear. Int. J. Dev. Neurosci. 15, 523–540 - PubMed
-
- Bernhagen J., Krohn R., Lue H., Gregory J. L., Zernecke A., Koenen R. R., Dewor M., Georgiev I., Schober A., Leng L., et al. (2007). MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 13, 587–596 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

