Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome
- PMID: 23172919
- PMCID: PMC3609577
- DOI: 10.2337/db12-1139
Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome
Abstract
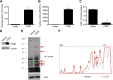
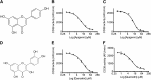
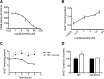
Metabolic syndrome is a growing health problem worldwide. It is therefore imperative to develop new strategies to treat this pathology. In the past years, the manipulation of NAD(+) metabolism has emerged as a plausible strategy to ameliorate metabolic syndrome. In particular, an increase in cellular NAD(+) levels has beneficial effects, likely because of the activation of sirtuins. Previously, we reported that CD38 is the primary NAD(+)ase in mammals. Moreover, CD38 knockout mice have higher NAD(+) levels and are protected against obesity and metabolic syndrome. Here, we show that CD38 regulates global protein acetylation through changes in NAD(+) levels and sirtuin activity. In addition, we characterize two CD38 inhibitors: quercetin and apigenin. We show that pharmacological inhibition of CD38 results in higher intracellular NAD(+) levels and that treatment of cell cultures with apigenin decreases global acetylation as well as the acetylation of p53 and RelA-p65. Finally, apigenin administration to obese mice increases NAD(+) levels, decreases global protein acetylation, and improves several aspects of glucose and lipid homeostasis. Our results show that CD38 is a novel pharmacological target to treat metabolic diseases via NAD(+)-dependent pathways.
Figures





Similar articles
-
CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats.Aging (Albany NY). 2020 Jun 7;12(12):11325-11336. doi: 10.18632/aging.103410. Epub 2020 Jun 7. Aging (Albany NY). 2020. PMID: 32507768 Free PMC article.
-
Inhibition of CD38 and supplementation of nicotinamide riboside ameliorate lipopolysaccharide-induced microglial and astrocytic neuroinflammation by increasing NAD.J Neurochem. 2021 Jul;158(2):311-327. doi: 10.1111/jnc.15367. Epub 2021 May 9. J Neurochem. 2021. PMID: 33871064 Free PMC article.
-
Apigenin Alleviates Oxidative Stress-Induced Cellular Senescence via Modulation of the SIRT1-NAD[Formula: see text]-CD38 Axis.Am J Chin Med. 2021;49(5):1235-1250. doi: 10.1142/S0192415X21500592. Epub 2021 May 27. Am J Chin Med. 2021. PMID: 34049472
-
The Pharmacology of CD38/NADase: An Emerging Target in Cancer and Diseases of Aging.Trends Pharmacol Sci. 2018 Apr;39(4):424-436. doi: 10.1016/j.tips.2018.02.001. Epub 2018 Feb 23. Trends Pharmacol Sci. 2018. PMID: 29482842 Free PMC article. Review.
-
CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions.Curr Pharm Des. 2009;15(1):57-63. doi: 10.2174/138161209787185788. Curr Pharm Des. 2009. PMID: 19149603 Free PMC article. Review.
Cited by
-
Immunometabolism: signaling pathways, homeostasis, and therapeutic targets.MedComm (2020). 2024 Nov 3;5(11):e789. doi: 10.1002/mco2.789. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39492834 Free PMC article. Review.
-
CD38 is methylated in prostate cancer and regulates extracellular NAD.Cancer Metab. 2018 Sep 21;6:13. doi: 10.1186/s40170-018-0186-3. eCollection 2018. Cancer Metab. 2018. PMID: 30258629 Free PMC article.
-
KL1333, a Novel NAD+ Modulator, Improves Energy Metabolism and Mitochondrial Dysfunction in MELAS Fibroblasts.Front Neurol. 2018 Jul 5;9:552. doi: 10.3389/fneur.2018.00552. eCollection 2018. Front Neurol. 2018. PMID: 30026729 Free PMC article.
-
Identification of Genetic Variations in the NAD-Related Pathways for Patients with Major Depressive Disorder: A Case-Control Study in Taiwan.J Clin Med. 2022 Jun 23;11(13):3622. doi: 10.3390/jcm11133622. J Clin Med. 2022. PMID: 35806906 Free PMC article.
-
Interplay between compartmentalized NAD+ synthesis and consumption: a focus on the PARP family.Genes Dev. 2020 Mar 1;34(5-6):254-262. doi: 10.1101/gad.335109.119. Epub 2020 Feb 6. Genes Dev. 2020. PMID: 32029457 Free PMC article. Review.
References
-
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA 2004;291:2847–2850 - PubMed
-
- Spiegel AM, Alving BM. Executive summary of the Strategic Plan for National Institutes of Health Obesity Research. Am J Clin Nutr 2005;82(Suppl.):211S–214S - PubMed
-
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–1428 - PubMed
-
- Barbosa MT, Soares SM, Novak CM, et al. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J 2007;21:3629–3639 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

