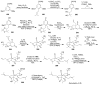
Synthetic Efforts Toward [3.3.1] Bridged Bicyclic Phloroglucinol Natural Products
- PMID: 23172980
- PMCID: PMC3501273
- DOI: 10.1016/j.tet.2011.06.079
Synthetic Efforts Toward [3.3.1] Bridged Bicyclic Phloroglucinol Natural Products
Figures







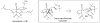
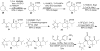


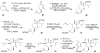

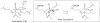

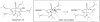




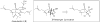

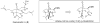

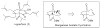
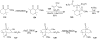



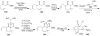


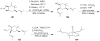


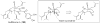


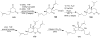






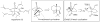




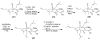


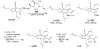
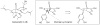
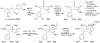


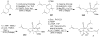






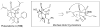


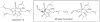


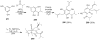




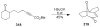
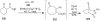
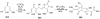
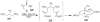

Similar articles
-
An efficient oxidative dearomatization-radical cyclization approach to symmetrically substituted bicyclic guttiferone natural products.Chem Commun (Camb). 2011 Jan 7;47(1):209-11. doi: 10.1039/c0cc01419b. Epub 2010 Jul 7. Chem Commun (Camb). 2011. PMID: 20607196 Free PMC article.
-
A short biomimetic approach to the fully functionalized bicyclic framework of type A acylphloroglucinols.Org Lett. 2009 Oct 1;11(19):4430-3. doi: 10.1021/ol901781n. Org Lett. 2009. PMID: 19739652
-
Recent advances in the synthesis of natural products containing the phloroglucinol motif.Nat Prod Rep. 2022 Sep 21;39(9):1766-1802. doi: 10.1039/d1np00077b. Nat Prod Rep. 2022. PMID: 35762867 Review.
-
Annulative Methods in the Synthesis of Complex Meroterpene Natural Products.Acc Chem Res. 2021 Feb 2;54(3):583-594. doi: 10.1021/acs.accounts.0c00781. Epub 2021 Jan 15. Acc Chem Res. 2021. PMID: 33448794
-
Structural and biological aspects of natural bridged macrobicyclic peptides from marine resources.Arch Pharm (Weinheim). 2021 Aug;354(8):e2100034. doi: 10.1002/ardp.202100034. Epub 2021 Apr 29. Arch Pharm (Weinheim). 2021. PMID: 33913195 Review.
Cited by
-
Total Synthesis of Hypersampsone M.J Am Chem Soc. 2024 Jul 17;146(28):18886-18891. doi: 10.1021/jacs.4c07007. Epub 2024 Jul 3. J Am Chem Soc. 2024. PMID: 38958271 Free PMC article.
-
Regiodivergent Photocyclization of Dearomatized Acylphloroglucinols: Asymmetric Syntheses of (-)-Nemorosone and (-)-6-epi-Garcimultiflorone A.J Am Chem Soc. 2019 Jul 17;141(28):11315-11321. doi: 10.1021/jacs.9b05600. Epub 2019 Jul 2. J Am Chem Soc. 2019. PMID: 31264859 Free PMC article.
-
Bioinspired Total Synthesis of Erectones A and B, and the Revised Structure of Hyperelodione D.Angew Chem Int Ed Engl. 2022 May 2;61(19):e202200420. doi: 10.1002/anie.202200420. Epub 2022 Mar 14. Angew Chem Int Ed Engl. 2022. PMID: 35225410 Free PMC article.
-
Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins.Chem Rev. 2016 Aug 10;116(15):8912-9000. doi: 10.1021/acs.chemrev.6b00334. Epub 2016 Jul 27. Chem Rev. 2016. PMID: 27461578 Free PMC article.
-
Total synthesis of (±)-7-epi-nemorosone.Org Lett. 2012 Apr 6;14(7):1796-9. doi: 10.1021/ol300386t. Epub 2012 Mar 26. Org Lett. 2012. PMID: 22449198 Free PMC article.
References
-
- Beerhues L. Phytochemistry. 2006;67:2201. - PubMed
-
-
Ciochina R, Grossman RB. Chem Rev. 2006;106:3963.Most recently, a microreview focused on the few completed total synthesis of member of this family was published (in Japanese): Tsukano C, Siegel DS, Danishefsky SJ. Yuki Gosey Kagaku Kyokai. 2010;68:592.For a more recent short synthetic discussion of some of these routes, see: Singh IP, Sidana J, Bharate SB, Foley W. J Nat Prod Rep. 2010;27:393.
-
-
- Fukuyama Y, Minami H, Kuwayama A. Phytochemistry. 1998;48:853.
-
- Cuesta-Rubio O, Velez-Castro H, Frontana-Uribe BA, Cardenas J. Phytochemistry. 2001;57:279. - PubMed
-
- de Oliveira CMA, Porto AM, Bittrich V, Vencato I, Marsaioli AJ. Tetrahedron Lett. 1996;37:6427.
Grants and funding
LinkOut - more resources
Full Text Sources
