Evaluation of immunoprotective activity of six leptospiral proteins in the hamster model of leptospirosis
- PMID: 23173023
- PMCID: PMC3502890
- DOI: 10.2174/1874285801206010079
Evaluation of immunoprotective activity of six leptospiral proteins in the hamster model of leptospirosis
Abstract
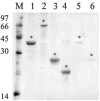
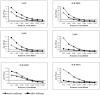
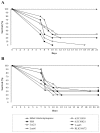
Leptospirosis is a worldwide zoonosis caused by pathogenic Leptospira. The whole-genome sequence of L. interrogans serovar Copenhageni together with bioinformatics tools represent a great opportunity to search for novel antigen candidates that could be used as subunit vaccine against leptospirosis. We focused on six genes encoding for conserved hypothetical proteins predicted to be exported to the outer membrane. The genes were amplified by PCR from Leptospira interrogans genomic DNA and were cloned and expressed in Escherichia coli. The recombinant proteins tagged with N-terminal hexahistidine were purified by metal-charged chromatography. The immunization of hamsters followed by challenge with lethal dose of virulent strain of Leptospira showed that the recombinant proteins Lsa21, Lsa66 and rLIC11030 elicited partial protection to animals. These proteins could be used combined or in a mixture with novel adjuvants in order to improve their effectiveness.
Keywords: Leptospira interrogans; leptospirosis; recombinant protein; vaccine..
Figures

Similar articles
-
Characterization of leptospiral proteins that afford partial protection in hamsters against lethal challenge with Leptospira interrogans.J Med Microbiol. 2010 Sep;59(Pt 9):1005-1015. doi: 10.1099/jmm.0.021485-0. Epub 2010 Jun 17. J Med Microbiol. 2010. PMID: 20558583
-
Whole-genome analysis of Leptospira interrogans to identify potential vaccine candidates against leptospirosis.FEMS Microbiol Lett. 2005 Mar 15;244(2):305-13. doi: 10.1016/j.femsle.2005.02.004. FEMS Microbiol Lett. 2005. PMID: 15766783
-
Challenges for the development of a universal vaccine against leptospirosis revealed by the evaluation of 22 vaccine candidates.Front Cell Infect Microbiol. 2022 Oct 7;12:940966. doi: 10.3389/fcimb.2022.940966. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36275031 Free PMC article.
-
Immunoprotective properties of recombinant LigA and LigB in a hamster model of acute leptospirosis.PLoS One. 2017 Jul 13;12(7):e0180004. doi: 10.1371/journal.pone.0180004. eCollection 2017. PLoS One. 2017. PMID: 28704385 Free PMC article.
-
Challenges and Strategies for Developing Recombinant Vaccines against Leptospirosis: Role of Expression Platforms and Adjuvants in Achieving Protective Efficacy.Pathogens. 2023 May 31;12(6):787. doi: 10.3390/pathogens12060787. Pathogens. 2023. PMID: 37375478 Free PMC article. Review.
Cited by
-
Immunoprotective Activity Induced by Leptospiral Outer Membrane Proteins in Hamster Model of Acute Leptospirosis.Front Immunol. 2020 Oct 30;11:568694. doi: 10.3389/fimmu.2020.568694. eCollection 2020. Front Immunol. 2020. PMID: 33193344 Free PMC article.
-
Evaluation of Lsa46 and Lsa77 Leptospiral Proteins for Their Immunoprotective Activities in Hamster Model of Leptospirosis.Biomed Res Int. 2018 Jun 10;2018:1813745. doi: 10.1155/2018/1813745. eCollection 2018. Biomed Res Int. 2018. PMID: 29984227 Free PMC article.
-
Induction of boosted immune response in mice by leptospiral surface proteins expressed in fusion with DnaK.Biomed Res Int. 2014;2014:564285. doi: 10.1155/2014/564285. Epub 2014 Jul 6. Biomed Res Int. 2014. PMID: 25110682 Free PMC article.
-
Revisiting the Development of Vaccines Against Pathogenic Leptospira: Innovative Approaches, Present Challenges, and Future Perspectives.Front Immunol. 2022 Jan 3;12:760291. doi: 10.3389/fimmu.2021.760291. eCollection 2021. Front Immunol. 2022. PMID: 35046936 Free PMC article. Review.
-
Current immunological and molecular tools for leptospirosis: diagnostics, vaccine design, and biomarkers for predicting severity.Ann Clin Microbiol Antimicrob. 2015 Jan 16;14:2. doi: 10.1186/s12941-014-0060-2. Ann Clin Microbiol Antimicrob. 2015. PMID: 25591623 Free PMC article. Review.
References
-
- Faine S, Adler B, Bolin C, Perolat P. Leptospira and Leptospirosis. Melbourne, Australia: Medi Sci. 1999.
-
- Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–71. - PubMed
-
- Ko AI, Galvao Reis M, Ribeiro Dourado CM, Johnson WD, Jr, Riley LW. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet. 1999;354:820–5. - PubMed
-
- Plank R, Dean D. Overview of the epidemiology, microbiology, and pathogenesis of Leptospira spp. in humans. Microbes Infect. 2000;2:1265–76. - PubMed
-
- de la Pena-Moctezuma A, Bulach DM, Kalambaheti T, Adler B. Comparative analysis of the LPS biosynthetic loci of the genetic subtypes of serovar Hardjo: Leptospira interrogans subtype Hardjoprajitno and Leptospira borgpetersenii subtype Hardjobovis. FEMS Microbiol Lett. 1999;177:319–26. - PubMed
LinkOut - more resources
Full Text Sources
