Osteoclasts in multiple myeloma are derived from Gr-1+CD11b+myeloid-derived suppressor cells
- PMID: 23173040
- PMCID: PMC3500251
- DOI: 10.1371/journal.pone.0048871
Osteoclasts in multiple myeloma are derived from Gr-1+CD11b+myeloid-derived suppressor cells
Abstract
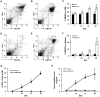
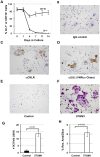
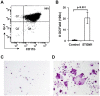
Osteoclasts play a key role in the development of cancer-associated osteolytic lesions. The number and activity of osteoclasts are often enhanced by tumors. However, the origin of osteoclasts is unknown. Myeloid-derived suppressor cells (MDSCs) are one of the pre-metastatic niche components that are induced to expand by tumor cells. Here we show that the MDSCs can differentiate into mature and functional osteoclasts in vitro and in vivo. Inoculation of 5TGM1-GFP myeloma cells into C57BL6/KaLwRij mice led to a significant expansion of MDSCs in blood, spleen, and bone marrow over time. When grown in osteoclastogenic media in vitro, MDSCs from tumor-challenged mice displayed 14 times greater potential to differentiate into mature and functional osteoclasts than those from non-tumor controls. Importantly, MDSCs from tumor-challenged LacZ transgenic mice differentiated into LacZ+osteoclasts in vivo. Furthermore, a significant increase in tumor burden and bone loss accompanied by increased number of osteoclasts was observed in mice co-inoculated with tumor-challenged MDSCs and 5TGM1 cells compared to the control animals received 5TGM1 cells alone. Finally, treatment of MDSCs from myeloma-challenged mice with Zoledronic acid (ZA), a potent inhibitor of bone resorption, inhibited the number of osteoclasts formed in MDSC cultures and the expansion of MDSCs and bone lesions in mice. Collectively, these data provide in vitro and in vivo evidence that tumor-induced MDSCs exacerbate cancer-associated bone destruction by directly serving as osteoclast precursors.
Conflict of interest statement
Figures





References
-
- Kaplan RN, Psaila B, Lyden D (2007) Niche-to-niche migration of bone-marrow-derived cells. Trends in molecular medicine 13: 72–81. - PubMed
-
- Kaplan RN, Psaila B, Lyden D (2006) Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer metastasis reviews 25: 521–529. - PubMed
-
- Psaila B, Kaplan RN, Port ER, Lyden D (2006) Priming the ‘soil’ for breast cancer metastasis: the pre-metastatic niche. Breast disease 26: 65–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

