An N-heterocyclic amine chelate capable of antioxidant capacity and amyloid disaggregation
- PMID: 23173072
- PMCID: PMC3503443
- DOI: 10.1021/cn300060v
An N-heterocyclic amine chelate capable of antioxidant capacity and amyloid disaggregation
Abstract
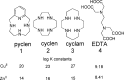
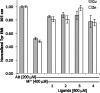
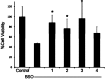
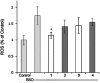
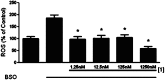
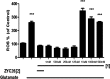
Alzheimer's disease is a neurodegenerative disorder characterized by the development of intracellular neurofibrillary tangles, deposition of extracellular amyloid beta (Aβ) plaques, along with a disruption of transition metal ion homeostasis in conjunction with oxidative stress. Spectroscopic, transmission electron microscopy, and scanning electron microscopy imaging studies show that 1 (pyclen) is capable of both preventing and disrupting Cu(2+) induced AB(1-40) aggregation. The pyridine backbone of 1 engenders antioxidant capacity, as shown by cellular DCFH-DA (dichlorodihydrofluorescein diacetate) assay in comparison to other N-heterocyclic amines lacking this aromatic feature. Finally, 1 prevents cell death induced by oxidative stress as shown by the Calcein AM assay. The results are supported using density functional theory studies which show that the pyridine backbone is responsible for the antioxidant capacity observed.
Figures


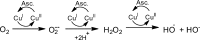


Similar articles
-
Zn(II)- and Cu(II)-induced non-fibrillar aggregates of amyloid-beta (1-42) peptide are transformed to amyloid fibrils, both spontaneously and under the influence of metal chelators.J Neurochem. 2009 Sep;110(6):1784-95. doi: 10.1111/j.1471-4159.2009.06269.x. Epub 2009 Jul 8. J Neurochem. 2009. PMID: 19619132
-
Management of oxidative stress and other pathologies in Alzheimer's disease.Arch Toxicol. 2019 Sep;93(9):2491-2513. doi: 10.1007/s00204-019-02538-y. Epub 2019 Aug 22. Arch Toxicol. 2019. PMID: 31440798 Review.
-
Multiple function fluorescein probe performs metal chelation, disaggregation, and modulation of aggregated Aβ and Aβ-Cu complex.ACS Chem Neurosci. 2015 Nov 18;6(11):1880-91. doi: 10.1021/acschemneuro.5b00205. Epub 2015 Sep 14. ACS Chem Neurosci. 2015. PMID: 26332658
-
Two nitrogen-containing ligands as inhibitors of metal-induced amyloid β-peptide aggregation.CNS Neurol Disord Drug Targets. 2014 Feb;13(1):166-72. doi: 10.2174/18715273113129990076. CNS Neurol Disord Drug Targets. 2014. PMID: 23844690
-
Polyphenols as Potential Metal Chelation Compounds Against Alzheimer's Disease.J Alzheimers Dis. 2021;82(s1):S335-S357. doi: 10.3233/JAD-200185. J Alzheimers Dis. 2021. PMID: 32568200 Free PMC article. Review.
Cited by
-
A potent antioxidant small molecule aimed at targeting metal-based oxidative stress in neurodegenerative disorders.Chem Commun (Camb). 2013 Apr 4;49(26):2712-4. doi: 10.1039/c2cc36808k. Chem Commun (Camb). 2013. PMID: 23437435 Free PMC article.
-
Advances in Multi-Functional Ligands and the Need for Metal-Related Pharmacology for the Management of Alzheimer Disease.Front Pharmacol. 2018 Nov 15;9:1247. doi: 10.3389/fphar.2018.01247. eCollection 2018. Front Pharmacol. 2018. PMID: 30498443 Free PMC article. Review.
-
Stabilisation of Exotic Tribromide (Br3-) Anions via Supramolecular Interaction with A Tosylated Macrocyclic Pyridinophane. A Serendipitous Case.Molecules. 2020 Jul 10;25(14):3155. doi: 10.3390/molecules25143155. Molecules. 2020. PMID: 32664239 Free PMC article.
-
Therapeutic Role of Heterocyclic Compounds in Neurodegenerative Diseases: Insights from Alzheimer's and Parkinson's Diseases.Neurol Int. 2025 Feb 7;17(2):26. doi: 10.3390/neurolint17020026. Neurol Int. 2025. PMID: 39997657 Free PMC article. Review.
-
Protein Formulations Containing Polysorbates: Are Metal Chelators Needed at All?Antioxidants (Basel). 2020 May 20;9(5):441. doi: 10.3390/antiox9050441. Antioxidants (Basel). 2020. PMID: 32443662 Free PMC article.
References
-
- (2011) Alzheimer’s Disease Facts and Figures, Alzheimer’s & Dementia, Alzheimer’s Association, p 7. - PubMed
-
- Wenk G. L. (2003) Neuropathologic changes in Alzheimer’s disease. J. Clin. Psychiatry 64, 7–10. - PubMed
-
- Wolk D. A.; Grachev I. D.; Buckley C.; Kazi H.; Grady M. S.; Trojanowski J. Q.; Hamilton R. H.; Sherwin P.; McLain R.; Arnold. S. E. (2011) Association Between In Vivo Fluorine 18-Labeled Flutemetamol Amyloid Positron Emission Tomography Imaging and In Vivo Cerebral Cortical Histopathology. Arch. Neurol. 68, 1398–1403. - PMC - PubMed
-
- Fleisher S.; Chen K.; Liu X.; Roontiva A.; Thiyyagura P.; Ayutyanont N.; Joshi A. D.; Clark C. M.; Mintun M. A.; Pontecorvo M. J.; Doraiswamy P. M.; Johnson K. A.; Skovronsky D. M.; Reiman E. M. (2011) Using Positron Emission Tomography and Florbetapir F 18 to Image Cortical Amyloid in Patients With Mild Cognitive Impairment or Dementia Due to Alzheimer Disease. Arch. Neurol. 68, 1461–1466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

