Therapeutic algorithms for chronic hepatitis C in the DAA era during the current economic crisis: whom to treat? How to treat? When to treat?
- PMID: 23173606
- PMCID: PMC3495629
- DOI: 10.1186/1471-2334-12-S2-S3
Therapeutic algorithms for chronic hepatitis C in the DAA era during the current economic crisis: whom to treat? How to treat? When to treat?
Abstract
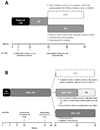
The advent of triple therapy (TT) with first-generation protease inhibitors boceprevir (BOC) and telaprevir (TVR) in addition to pegylated interferon and ribavirin resulted in a significant gain in terms of sustained virological response (SVR) when treating naive or previous treated patients with genotype 1 (G1) chronic hepatitis C (CHC). This gain is partly balanced by the increased complexity of treatment and by the raised costs and risks of therapy, making necessary to optimize the indication to TT.Specifically, the identification of patient needing to TT over DT, the choice of the more correct therapeutic approach according to baseline and on treatment SVR predictors, and the timing of antiviral treatment, appear key issues to evaluate when considering TVR or BOC-based therapies.Along this line, further efforts aimed to optimize the current TT regimens are still needed, especially in under-represented groups of patients in phase 3 studies such as those with cirrhosis, where post-marketing data are giving interesting evidences.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

