Upregulation of mesencephalic astrocyte-derived neurotrophic factor in glial cells is associated with ischemia-induced glial activation
- PMID: 23173607
- PMCID: PMC3576245
- DOI: 10.1186/1742-2094-9-254
Upregulation of mesencephalic astrocyte-derived neurotrophic factor in glial cells is associated with ischemia-induced glial activation
Abstract
Background: Mesencephalic astrocyte-derived neurotrophic factor (MANF), a 20 kDa secreted protein, was originally derived from a rat mesencephalic type-1 astrocyte cell line. MANF belongs to a novel evolutionally conserved family of neurotrophic factors along with conserved dopamine neurotrophic factor. In recent years, ever-increasing evidence has shown that both of them play a remarkable protective role against various injuries to neurons in vivo or in vitro. However, the characteristics of MANF expression in the different types of glial cells, especially in astrocytes, remain unclear.
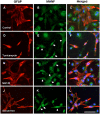
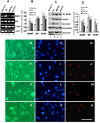
Methods: The model of focal cerebral ischemia was induced by rat middle cerebral artery occlusion. Double-labeled immunofluorescent staining was used to identify the types of neural cells expressing MANF. Primarily cultured glial cells were used to detect the response of glial cells to endoplasmic reticulum stress stimulation. Propidium iodide staining was used to determine dead cells. Reverse transcription PCR and western blotting were used to detect the levels of mRNA and proteins.
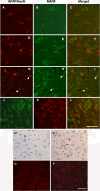
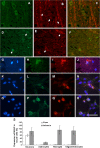
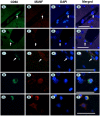
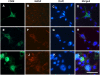
Results: We found that MANF was predominantly expressed in neurons in both normal and ischemic cortex. Despite its name, MANF was poorly expressed in glial cells, including astrocytes, in normal brain tissue. However, the expression of MANF was upregulated in the glial cells under focal cerebral ischemia, including the astrocytes. This expression was also induced by several endoplasmic reticulum stress inducers and nutrient deprivation in cultured primary glial cells. The most interesting phenomenon observed in this study was the pattern of MANF expression in the microglia. The expression of MANF was closely associated with the morphology and state of microglia, accompanied by the upregulation of BIP/Grp78.
Conclusions: These results indicate that MANF expression was upregulated in the activated glial cells, which may contribute to the mechanism of ischemia-induced neural injury.
Figures

References
-
- Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, Durocher Y, Commissiong JW. MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J Mol Neurosci. 2003;20:173–188. doi: 10.1385/JMN:20:2:173. - DOI - PubMed
-
- Shridhar V, Rivard S, Shridhar R, Mullins C, Bostick L, Sakr W, Grignon D, Miller OJ, Smith DI. A gene from human chromosomal band 3p21.1 encodes a highly conserved arginine-rich protein and is mutated in renal cell carcinomas. Oncogene. 1996;12:1931–1939. - PubMed
-
- Shridhar R, Shridhar V, Rivard S, Siegfried JM, Pietraszkiewicz H, Ensley J, Pauley R, Grignon D, Sakr W, Miller OJ, Smith DI. Mutations in the arginine-rich protein gene, in lung, breast, and prostate cancers, and in squamous cell carcinoma of the head and neck. Cancer Res. 1996;56:5576–5578. - PubMed
-
- Evron E, Cairns P, Halachmi N, Ahrendt SA, Reed AL, Sidransky D. Normal polymorphism in the incomplete trinucleotide repeat of the arginine-rich protein gene. Cancer Res. 1997;57:2888–2889. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

