West Nile alternative open reading frame (N-NS4B/WARF4) is produced in infected West Nile Virus (WNV) cells and induces humoral response in WNV infected individuals
- PMID: 23173701
- PMCID: PMC3574045
- DOI: 10.1186/1743-422X-9-283
West Nile alternative open reading frame (N-NS4B/WARF4) is produced in infected West Nile Virus (WNV) cells and induces humoral response in WNV infected individuals
Abstract
Background: West Nile Virus (WNV) is a flavivirus that requires an efficient humoral and cellular host response for the control of neuroinvasive infection. We previously reported the existence of six alternative open reading frame proteins in WNV genome, one of which entitled WARF4 is exclusively restricted to the lineage I of the virus. WARF4 is able to elicit antibodies in WNV infected horses; however, there was no direct experimental proof of the existence of this novel protein. The purpose of this study was to demonstrate the in vitro production of WARF4 protein following WNV infection of cultured VERO cells and its immunity in WNV infected individuals.
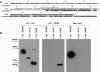
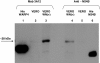
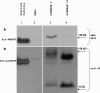
Results: We produced a monoclonal antibody against WARF4 protein (MAb 3A12) which detected the novel protein in WNV lineage I-infected, cultured VERO cells while it did not react with WNV lineage II infected cells. MAb 3A12 specificity to WARF4 protein was confirmed by its reactivity to only one peptide among four analyzed that cover the full WARF4 amino acids sequence. In addition, WARF4 protein was expressed in the late phase of WNV lineage I infection. Western blotting and bioinformatics analyses strongly suggest that the protein could be translated by programmed -1 ribosomal frameshifting process. Since WARF4 is embedded in the NS4B gene, we rename this novel protein N-NS4B/WARF4. Furthermore, serological analysis shows that N-NS4B/WARF4 is able to elicit antibodies in WNV infected individuals.
Conclusions: N-NS4B/WARF4 is the second Alternative Reading Frame (ARF) protein that has been demonstrated to be produced following WNV infection and might represent a novel tool for a better characterization of immune response in WNV infected individuals. Further serological as well as functional studies are required to characterize the function of the N-NS4B/WARF4 protein. Since the virus might actually make an extensive use of ARFs, it appears important to investigate the novel six ARF putative proteins of WNV.
Figures







References
-
- Monath TP. In: Virology. Fields BN, Knipe DM, editor. New York: Raven Press; 1990. Flaviviruses; pp. 763–814.
-
- Smithburn KC, Hughes TP, Burke AW, Paul JH. A Neurotropic Virus Isolated from the Blood of a Native of Uganda. Am J Trop Med. 1940;4:471–492.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

