Plasmodium serine hydroxymethyltransferase: indispensability and display of distinct localization
- PMID: 23173711
- PMCID: PMC3521198
- DOI: 10.1186/1475-2875-11-387
Plasmodium serine hydroxymethyltransferase: indispensability and display of distinct localization
Abstract
Background: Serine hydroxymethyltransferase (SHMT), a pyridoxal phosphate-dependent enzyme, plays a vital role in the de novo pyrimidine biosynthesis pathway in malaria parasites. Two genes have been identified in Plasmodium spp. encoding a cytosolic SHMT (cSHMT) and putative mitochondria SHMT (mSHMT), but their roles have not been fully investigated.
Methods: The presence of Plasmodium SHMT isoforms in the intra-erythrocytic stage was assessed based on their gene expression using reverse transcription PCR (RT-PCR). Localization studies of Plasmodium SHMT isoforms were performed by transfection of fluorescent-tagged gene constructs into P. falciparum and expressions of fluorescent fusion proteins in parasites were observed using a laser scanning confocal microscope. Genetic targeting through homologous recombination was used to study the essentiality of SHMT in Plasmodium spp.
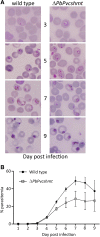
Results: Semi-quantitative RT-PCR revealed the expression of these two genes throughout intra-erythrocytic development. Localization studies using P. falciparum expressing fluorescent-tagged SHMT showed that PfcSHMT-red fluorescent fusion protein (PfcSHMT-DsRed) is localized in the cytoplasm, while PfmSHMT-green fluorescent fusion protein (PfmSHMT-GFP) co-localized with Mitotracker™-labelled mitochondria as predicted. The essentiality of plasmodial cSHMT was inferred from transfection experiments where recovery of viable knock-out parasites was not achieved, unless complemented with a functional equivalent copy of shmt.
Conclusions: Distinct compartment localizations of PfSHMT were observed between cytoplasmic and mitochondrial isoforms, and evidence was provided for the indispensable role of plasmodial cSHMT indicating it as a valid target for development of novel anti-malarials.
Figures



References
-
- Gero AM, O’Sullivan WJ. Purines and pyrimidines in malarial parasites. Blood Cells. 1990;16:467–484. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

