Effect of antioxidant supplementation on the total yield, oxidative stress levels, and multipotency of bone marrow-derived human mesenchymal stromal cells
- PMID: 23173771
- PMCID: PMC3589894
- DOI: 10.1089/ten.tea.2011.0700
Effect of antioxidant supplementation on the total yield, oxidative stress levels, and multipotency of bone marrow-derived human mesenchymal stromal cells
Abstract
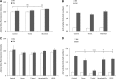
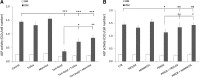
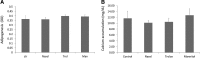
Bone marrow-derived multipotent mesenchymal stromal cells (MSCs) are the most frequently investigated cell type for potential regenerative strategies because they are relatively easy to isolate and are able to differentiate into several mesenchymal lineages. Unfortunately, during ex vivo culture, MSCs present gradual loss of differentiation potential and reduced clinical efficacy. Reactive oxygen species (ROS) are associated with oxidative damage and accumulate during MSC expansion. Because ROS are believed to be involved in the loss of multipotency, we hypothesized that compounds with antioxidant activity have the capacity to scavenge ROS, prevent cellular damage, and rescue culture-induced loss of multipotency. In this manuscript, we show that antioxidant supplementation can partially rescue the loss of alkaline phosphatase expression induced by oxidizing agents and increases the yield of hMSCs, when supplemented to a fresh bone marrow aspirate. Concomitantly, oxidative DNA damage and ROS levels in hMSCs were reduced by antioxidants. We conclude that antioxidant supplementation during MSC expansion reduces the DNA damage load and increases the MSC yield.
Figures


Similar articles
-
Conditioned Medium from Bone Marrow Mesenchymal Stem Cells Restored Oxidative Stress-Related Impaired Osteogenic Differentiation.Int J Mol Sci. 2021 Dec 15;22(24):13458. doi: 10.3390/ijms222413458. Int J Mol Sci. 2021. PMID: 34948255 Free PMC article.
-
Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro.Stem Cells. 2006 May;24(5):1226-35. doi: 10.1634/stemcells.2005-0117. Epub 2006 Jan 19. Stem Cells. 2006. PMID: 16424399
-
Atmospheric oxygen inhibits growth and differentiation of marrow-derived mouse mesenchymal stem cells via a p53-dependent mechanism: implications for long-term culture expansion.Stem Cells. 2012 May;30(5):975-87. doi: 10.1002/stem.1069. Stem Cells. 2012. PMID: 22367737 Free PMC article.
-
The emerging antioxidant paradigm of mesenchymal stem cell therapy.Stem Cells Transl Med. 2020 Sep;9(9):985-1006. doi: 10.1002/sctm.19-0446. Epub 2020 Jun 4. Stem Cells Transl Med. 2020. PMID: 32497410 Free PMC article. Review.
-
Mitigating Oxidative Stress in Perinatal Cells: A Critical Step toward an Optimal Therapeutic Use in Regenerative Medicine.Biomolecules. 2023 Jun 10;13(6):971. doi: 10.3390/biom13060971. Biomolecules. 2023. PMID: 37371551 Free PMC article. Review.
Cited by
-
Ginsenoside Rg1 protects human umbilical cord blood-derived stromal cells against tert-Butyl hydroperoxide-induced apoptosis through Akt-FoxO3a-Bim signaling pathway.Mol Cell Biochem. 2016 Oct;421(1-2):75-87. doi: 10.1007/s11010-016-2786-y. Epub 2016 Aug 13. Mol Cell Biochem. 2016. PMID: 27522666
-
Relationship between senescence in macaques and bone marrow mesenchymal stem cells and the molecular mechanism.Aging (Albany NY). 2019 Jan 23;11(2):590-614. doi: 10.18632/aging.101762. Aging (Albany NY). 2019. PMID: 30673631 Free PMC article.
-
The mechanism of (+) taxifolin's protective antioxidant effect for •OH-treated bone marrow-derived mesenchymal stem cells.Cell Mol Biol Lett. 2017 Dec 27;22:31. doi: 10.1186/s11658-017-0066-9. eCollection 2017. Cell Mol Biol Lett. 2017. PMID: 29299033 Free PMC article.
-
Anti-Obesity and Anti-Diabetic Effects of Ostericum koreanum (Ganghwal) Extract.Int J Mol Sci. 2024 Apr 30;25(9):4908. doi: 10.3390/ijms25094908. Int J Mol Sci. 2024. PMID: 38732125 Free PMC article.
-
Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: a possible mechanism of age related osteoporosis.Bone Res. 2015 Mar 17;3:15003. doi: 10.1038/boneres.2015.3. eCollection 2015. Bone Res. 2015. PMID: 26273536 Free PMC article.
References
-
- Kadiyala S. Culture-expanded, bone marrow-derived mesenchymal stem cells can regenerate a critical-sized segmental bone defect. Tissue Eng. 1997;3:173.
-
- Bruder S.P., et al. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin Orthop Relat Res. 1998;(355 Suppl):S247. - PubMed
-
- Almeida-Porada G., et al. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000;95:3620. - PubMed
-
- Tse W.T., et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389. - PubMed
-
- Ringden O., et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

